Prostate-specific membrane antigen as a target for cancer imaging and therapy
- PMID: 26213140
- PMCID: PMC4859214
Prostate-specific membrane antigen as a target for cancer imaging and therapy
Abstract
The prostate-specific membrane antigen (PSMA) is a molecular target whose use has resulted in some of the most productive work toward imaging and treating prostate cancer over the past two decades. A wide variety of imaging agents extending from intact antibodies to low-molecular-weight compounds permeate the literature. In parallel there is a rapidly expanding pool of antibody-drug conjugates, radiopharmaceutical therapeutics, small-molecule drug conjugates, theranostics and nanomedicines targeting PSMA. Such productivity is motivated by the abundant expression of PSMA on the surface of prostate cancer cells and within the neovasculature of other solid tumors, with limited expression in most normal tissues. Animating the field is a variety of small-molecule scaffolds upon which the radionuclides, drugs, MR-detectable species and nanoparticles can be placed with relative ease. Among those, the urea-based agents have been most extensively leveraged, with expanding clinical use for detection and more recently for radiopharmaceutical therapy of prostate cancer, with surprisingly little toxicity. PSMA imaging of other cancers is also appearing in the clinical literature, and may overtake FDG for certain indications. Targeting PSMA may provide a viable alternative or first-line approach to managing prostate and other cancers.
Conflict of interest statement
Figures



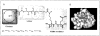



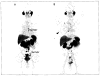

References
-
- Akhurst T, MacManus M, Hicks RJ. Lung cancer. PET clinics. 2015;10:147–58. - PubMed
-
- Meignan M, Itti E, Gallamini A, Younes A. FDG PET/CT imaging as a biomarker in lymphoma. Eur J Nucl Med Mol Imaging. 2015;42:623–33. - PubMed
-
- Schmidt T, Lordick F, Herrmann K, Ott K. Value of functional imaging by PET in esophageal cancer. J Natl Compr Canc Netw. 2015;13:239–47. - PubMed
-
- Fanti S, Nanni C, Ambrosini V, Gross MD, Rubello D, Farsad M. PET in genitourinary tract cancers. Q J Nucl Med Mol Imaging. 2007;51:260–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

