Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics
- PMID: 26213371
- PMCID: PMC4570933
- DOI: 10.1016/j.actbio.2015.07.034
Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics
Abstract
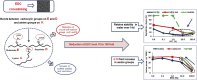
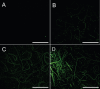
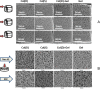
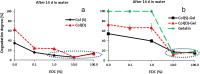
We provide evidence to show that the standard reactant concentrations used in tissue engineering to cross-link collagen-based scaffolds are up to 100 times higher than required for mechanical integrity in service, and stability against degradation in an aqueous environment. We demonstrate this with a detailed and systematic study by comparing scaffolds made from (a) collagen from two different suppliers, (b) gelatin (a partially denatured collagen) and (c) 50% collagen-50% gelatin mixtures. The materials were processed, using lyophilisation, to produce homogeneous, highly porous scaffolds with isotropic architectures and pore diameters ranging from 130 to 260 μm. Scaffolds were cross-linked using a carbodiimide treatment, to establish the effect of the variations in crosslinking conditions (down to very low concentrations) on the morphology, swelling, degradation and mechanical properties of the scaffolds. Carbodiimide concentration of 11.5mg/ml was defined as the standard (100%) and was progressively diluted down to 0.1%. It was found that 10-fold reduction in the carbodiimide content led to the significant increase (almost 4-fold) in the amount of free amine groups (primarily on collagen lysine residues) without compromising mechanics and stability in water of all resultant scaffolds. The importance of this finding is that, by reducing cross-linking, the corresponding cell-reactive carboxylate anions (collagen glutamate or aspartate residues) that are essential for integrin-mediated binding remain intact. Indeed, a 10-fold reduction in carbodiimide crosslinking resulted in near native-like cell attachment to collagen scaffolds. We have demonstrated that controlling the degree of cross-linking, and hence retaining native scaffold chemistry, offers a major step forward in the biological performance of collagen- and gelatin-based tissue engineering scaffolds.
Statement of significance: This work developed collagen and gelatine-based scaffolds with structural, material and biological properties suitable for use in myocardial tissue regeneration. The novelty and significance of this research consist in elucidating the effect of the composition, origin of collagen and crosslinking concentration on the scaffold physical and cell-binding characteristics. We demonstrate that the standard carbodiimide concentrations used to crosslink collagenous scaffolds are up to 100 times higher than required for mechanical integrity in service, and stability against dissolution. The importance of this finding is that, by reducing crosslinking, the corresponding cell-reactive carboxylate anions (essential for integrin-mediated binding) remain intact and the native scaffold chemistry is retained. This offers a major step forward in the biological performance of tissue engineered scaffolds.
Keywords: Collagen; Crosslinking; Gelatin; Scaffolds; Tissue engineering.
Copyright © 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures





References
-
- Chiu L.L.Y., Radisic M. Scaffolds with covalently immobilized VEGF and angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–241. - PubMed
-
- Lee C.H., Singla A., Lee Y.M. Biomedical applications of collagen. Int. J. Pharm. 2001;221:1–22. - PubMed
-
- Li R.-K., Jia Z.Q., Weisel R.D., Mickle D.A., Choi A., Yau T.M. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100:63–69. - PubMed
-
- Liu C., Xia Z., Czernuszka J.T. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007;85:1051–1064.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

