β-Neurexins Control Neural Circuits by Regulating Synaptic Endocannabinoid Signaling
- PMID: 26213384
- PMCID: PMC4709013
- DOI: 10.1016/j.cell.2015.06.056
β-Neurexins Control Neural Circuits by Regulating Synaptic Endocannabinoid Signaling
Abstract
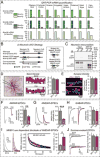
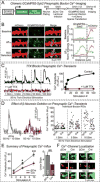
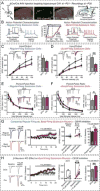
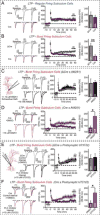
α- and β-neurexins are presynaptic cell-adhesion molecules implicated in autism and schizophrenia. We find that, although β-neurexins are expressed at much lower levels than α-neurexins, conditional knockout of β-neurexins with continued expression of α-neurexins dramatically decreased neurotransmitter release at excitatory synapses in cultured cortical neurons. The β-neurexin knockout phenotype was attenuated by CB1-receptor inhibition, which blocks presynaptic endocannabinoid signaling, or by 2-arachidonoylglycerol synthesis inhibition, which impairs postsynaptic endocannabinoid release. In synapses formed by CA1-region pyramidal neurons onto burst-firing subiculum neurons, presynaptic in vivo knockout of β-neurexins aggravated endocannabinoid-mediated inhibition of synaptic transmission and blocked LTP; presynaptic CB1-receptor antagonists or postsynaptic 2-arachidonoylglycerol synthesis inhibition again reversed this block. Moreover, conditional knockout of β-neurexins in CA1-region neurons impaired contextual fear memories. Thus, our data suggest that presynaptic β-neurexins control synaptic strength in excitatory synapses by regulating postsynaptic 2-arachidonoylglycerol synthesis, revealing an unexpected role for β-neurexins in the endocannabinoid-dependent regulation of neural circuits.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Neurotransmission: Sticking the brakes on.Nat Rev Neurosci. 2015 Sep;16(9):508-9. doi: 10.1038/nrn4013. Epub 2015 Aug 12. Nat Rev Neurosci. 2015. PMID: 26265507 No abstract available.
References
-
- Bang ML, Owczarek S. A matter of balance: role of neurexin and neuroligin at the synapse. Neurochemical research. 2013;38:1174–1189. - PubMed
-
- Behr J, Wozny C, Fidzinski P, Schmitz D. Synaptic plasticity in the subiculum. Prog Neurobiol. 2009;89:334–342. - PubMed
-
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

