Enhanced therapeutic efficacy of budesonide in experimental colitis with enzyme/pH dual-sensitive polymeric nanoparticles
- PMID: 26213469
- PMCID: PMC4509535
- DOI: 10.2147/IJN.S87816
Enhanced therapeutic efficacy of budesonide in experimental colitis with enzyme/pH dual-sensitive polymeric nanoparticles
Abstract
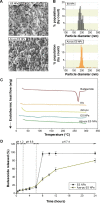
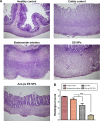
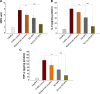
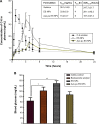
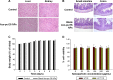
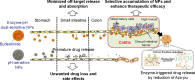
Current colon-targeted drug-delivery approaches for colitis therapy often utilize single pH-triggered systems, which are less reliable due to the variation of gut pH in individuals and in disease conditions. Herein, we prepared budesonide-loaded dual-sensitive nanoparticles using enzyme-sensitive azo-polyurethane and pH-sensitive methacrylate copolymer for the treatment of colitis. The therapeutic potential of the enzyme/pH dual-sensitive nanoparticles was evaluated using a rat colitis model and compared to single pH-triggered nanoparticles. Clinical activity scores, colon/body weight ratios, myeloperoxidase activity, and proinflammatory cytokine levels were markedly decreased by dual-sensitive nanoparticles compared to single pH-triggered nanoparticles and budesonide solution. Moreover, dual-sensitive nanoparticles accumulated selectively in inflamed segments of the colon. In addition, dual-sensitive nanoparticle plasma concentrations were lower than single pH-triggered nanoparticles, and no noticeable in vitro or in vivo toxicity was observed. Our results demonstrate that enzyme/pH dual-sensitive nanoparticles are an effective and safe colon-targeted delivery system for colitis therapy.
Keywords: azo-polyurethane; budesonide; colitis; colon-targeted nanoparticles; methacrylate copolymer.
Figures


References
-
- Seow CH, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;3:CD000296. - PubMed
-
- Nakase H, Okazaki K, Tabata Y, et al. Development of an oral drug delivery system targeting immune-regulating cells in experimental inflammatory bowel disease: a new therapeutic strategy. J Pharmacol Exp Ther. 2000;292(1):15–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

