Role of apolipoprotein E in neurodegenerative diseases
- PMID: 26213471
- PMCID: PMC4509527
- DOI: 10.2147/NDT.S84266
Role of apolipoprotein E in neurodegenerative diseases
Abstract
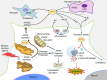
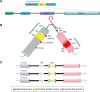
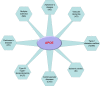
Apolipoprotein E (APOE) is a lipid-transport protein abundantly expressed in most neurons in the central nervous system. APOE-dependent alterations of the endocytic pathway can affect different functions. APOE binds to cell-surface receptors to deliver lipids and to the hydrophobic amyloid-β peptide, regulating amyloid-β aggregations and clearances in the brain. Several APOE isoforms with major structural differences were discovered and shown to influence the brain lipid transport, glucose metabolism, neuronal signaling, neuroinflammation, and mitochondrial function. This review will summarize the updated research progress on APOE functions and its role in Alzheimer's disease, Parkinson's disease, cardiovascular diseases, multiple sclerosis, type 2 diabetes mellitus, Type III hyperlipoproteinemia, vascular dementia, and ischemic stroke. Understanding the mutations in APOE, their structural properties, and their isoforms is important to determine its role in various diseases and to advance the development of therapeutic strategies. Targeting APOE may be a potential approach for diagnosis, risk assessment, prevention, and treatment of various neurodegenerative and cardiovascular diseases in humans.
Keywords: apolipoprotein E; diseases; pathogenesis.
Figures
References
-
- Rall SC, Jr, Weisgraber KH, Mahley RW. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982;257(8):4171–4178. - PubMed
-
- Mahley RW, Huang Y. Apolipoprotein E: from atherosclerosis to Alzheimer’s disease and beyond. Curr Opin Lipidol. 1999;10(3):207–217. - PubMed
-
- Mahley RW, Rall SC. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. - PubMed
-
- Emi M, Wu LL, Robertson MA, et al. Genotyping and sequence analysis of apolipoprotein Eisoforms. Genomics. 1988;3(4):373–379. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

