Delivery of iPS-NPCs to the Stroke Cavity within a Hyaluronic Acid Matrix Promotes the Differentiation of Transplanted Cells
- PMID: 26213530
- PMCID: PMC4512237
- DOI: 10.1002/adfm.201401483
Delivery of iPS-NPCs to the Stroke Cavity within a Hyaluronic Acid Matrix Promotes the Differentiation of Transplanted Cells
Abstract
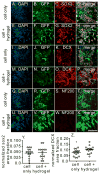
Stroke is the leading cause of adult disability with ~80% being ischemic. Stem cell transplantation has been shown to improve functional recovery. However, the overall survival and differentiation of these cells is still low. The infarct cavity is an ideal location for transplantation as it is directly adjacent to the highly plastic peri-infarct region. Direct transplantation of cells near the infarct cavity has resulted in low cell viability. Here we deliver neural progenitor cells derived from induce pluripotent stem cells (iPS-NPC) to the infarct cavity of stroked mice encapsulated in a hyaluronic acid hydrogel matrix to protect the cells. To improve the overall viability of transplanted cells, each step of the transplantation process was optimized. Hydrogel mechanics and cell injection parameters were investigated to determine their effects on the inflammatory response of the brain and cell viability, respectively. Using parameters that balanced the desire to keep surgery invasiveness minimal and cell viability high, iPS-NPCs were transplanted to the stroke cavity of mice encapsulated in buffer or the hydrogel. While the hydrogel did not promote stem cell survival one week post-transplantation, it did promote differentiation of the neural progenitor cells to neuroblasts.
Keywords: Hyaluronic acid; Hydrogel; Induced pluripotent stem cells; Stroke.
Figures





References
-
- Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Stroke. 1996;27:1459. - PubMed
-
- Broderick JP. Stroke. 2004;35:205. - PubMed
-
- Donnan GA, Fisher M, Macleod M, Davis SM. Lancet. 2008;371:1612. - PubMed
-
- Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, Reymann KG, Dihne M. Brain. 2006;129:3238. - PubMed
-
- Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, Hovatta O, Jolkkonen J. European Journal of Neuroscience. 2009;29:562. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
