The dependences of osteocyte network on bone compartment, age, and disease
- PMID: 26213632
- PMCID: PMC4511381
- DOI: 10.1038/boneres.2015.9
The dependences of osteocyte network on bone compartment, age, and disease
Abstract
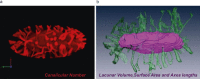
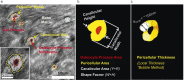
Osteocytes, the most abundant bone cells, form an interconnected network in the lacunar-canalicular pore system (LCS) buried within the mineralized matrix, which allows osteocytes to obtain nutrients from the blood supply, sense external mechanical signals, and communicate among themselves and with other cells on bone surfaces. In this study, we examined key features of the LCS network including the topological parameter and the detailed structure of individual connections and their variations in cortical and cancellous compartments, at different ages, and in two disease conditions with altered mechanosensing (perlecan deficiency and diabetes). LCS network showed both topological stability, in terms of conservation of connectivity among osteocyte lacunae (similar to the "nodes" in a computer network), and considerable variability the pericellular annular fluid gap surrounding lacunae and canaliculi (similar to the "bandwidth" of individual links in a computer network). Age, in the range of our study (15-32 weeks), affected only the pericellular fluid annulus in cortical bone but not in cancellous bone. Diabetes impacted the spacing of the lacunae, while the perlecan deficiency had a profound influence on the pericellular fluid annulus. The LCS network features play important roles in osteocyte signaling and regulation of bone growth and adaptation.
Figures



References
-
- Cowin SC. Bone Mechanics Handbook, 2nd edn. Boca Raton, FL: CRC Press, 2001.
-
- Lean JM, Jagger CJ, Chambers TJ, Chow JW. Increased insulin-like growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. Am J Physiol 1995; 268: E318–E327. - PubMed
-
- Forwood MR, Kelly WL, Worth NF. Localisation of prostaglandin endoperoxide H synthase (PGHS)-1 and PGHS-2 in bone following mechanical loading in vivo. Anat Rec 1998; 252: 580–586. - PubMed
-
- Terai K, Takano-Yamamoto T, Ohba Y et al. Role of osteopontin in bone remodeling caused by mechanical stress. J Bone Miner Res 1999; 14: 839–849. - PubMed
-
- Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature 2003; 424: 389. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

