Dysregulated intrahepatic CD4+ T-cell activation drives liver inflammation in ileitis-prone SAMP1/YitFc mice
- PMID: 26213712
- PMCID: PMC4511857
- DOI: 10.1016/j.jcmgh.2015.05.007
Dysregulated intrahepatic CD4+ T-cell activation drives liver inflammation in ileitis-prone SAMP1/YitFc mice
Abstract
Background and aims: Liver inflammation is a common extraintestinal manifestation of inflammatory bowel disease (IBD); however, whether liver involvement is a consequence of a primary intestinal defect or results from alternative pathogenic processes remains unclear. Therefore, we sought to determine the potential pathogenic mechanism(s) of concomitant liver inflammation in an established murine model of IBD.
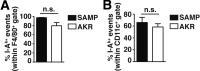
Methods: Liver inflammation and immune cell subsets were characterized in ileitis-prone SAMP1/YitFc (SAMP) and AKR/J (AKR) control mice, lymphocyte-depleted SAMP (SAMPxRag-1-/-), and immunodeficient SCID recipient mice receiving SAMP or AKR donor CD4+ T-cells. Proliferation and suppressive capacity of CD4+ T-effector (Teff) and T-regulatory (Treg) cells from gut-associated lymphoid tissue (GALT) and livers of SAMP and AKR mice were measured.
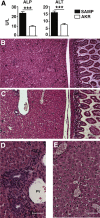
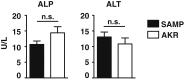
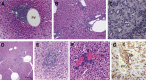
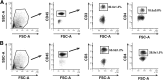
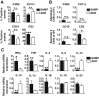
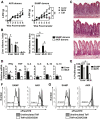
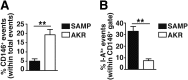
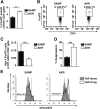
Results: Surprisingly, prominent inflammation was detected in 4-wk-old SAMP livers, prior to histologic evidence of ileitis, while both disease phenotypes were absent in age-matched AKRs. SAMP liver disease was characterized by abundant infiltration of lymphocytes, required for hepatic inflammation to occur, a Th1-skewed environment, and phenotypically-activated CD4+ T-cells. SAMP intrahepatic CD4+ T-cells also had the ability to induce liver and ileal inflammation when adoptively transferred into SCID recipients, whereas GALT-derived CD4+ T-cells produced milder ileitis, but not liver inflammation. Interestingly, SAMP intrahepatic CD4+ Teff cells showed increased proliferation compared to both SAMP GALT- and AKR liver-derived CD4+ Teff cells, while SAMP intrahepatic Tregs were decreased among CD4+ T-cells and impaired in in vitro suppressive function compared to AKR.
Conclusions: Activated intrahepatic CD4+ T-cells induce liver inflammation and contribute to experimental ileitis via locally-impaired hepatic immunosuppressive function.
Keywords: IBD-associated liver inflammation; SAMP1/YitFc mice; hepatic CD4+ T-cells; liver sinusoidal endothelial cells; regulatory T-cells.
Figures



Similar articles
-
Expanded B cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease.J Clin Invest. 2004 Aug;114(3):389-98. doi: 10.1172/JCI20855. J Clin Invest. 2004. PMID: 15286805 Free PMC article.
-
Death Receptor 3 Signaling Controls the Balance between Regulatory and Effector Lymphocytes in SAMP1/YitFc Mice with Crohn's Disease-Like Ileitis.Front Immunol. 2018 Mar 1;9:362. doi: 10.3389/fimmu.2018.00362. eCollection 2018. Front Immunol. 2018. PMID: 29545797 Free PMC article.
-
Epithelial-specific Toll-like Receptor (TLR)5 Activation Mediates Barrier Dysfunction in Experimental Ileitis.Inflamm Bowel Dis. 2017 Mar;23(3):392-403. doi: 10.1097/MIB.0000000000001035. Inflamm Bowel Dis. 2017. PMID: 28146004
-
Pathogenesis of gastritis in ileitis-prone SAMP1/Yit mice.Keio J Med. 2011;60(2):65-8. doi: 10.2302/kjm.60.65. Keio J Med. 2011. PMID: 21720202 Review.
-
SAMP1/YitFc mouse strain: a spontaneous model of Crohn's disease-like ileitis.Inflamm Bowel Dis. 2011 Dec;17(12):2566-84. doi: 10.1002/ibd.21638. Epub 2011 May 6. Inflamm Bowel Dis. 2011. PMID: 21557393 Free PMC article. Review.
Cited by
-
Uncovering Pathogenic Mechanisms of Inflammatory Bowel Disease Using Mouse Models of Crohn's Disease-Like Ileitis: What is the Right Model?Cell Mol Gastroenterol Hepatol. 2017 Mar 6;4(1):19-32. doi: 10.1016/j.jcmgh.2017.02.010. eCollection 2017 Jul. Cell Mol Gastroenterol Hepatol. 2017. PMID: 28560286 Free PMC article. Review.
-
Endoplasmic Reticulum Stress Regulates Hepatic Bile Acid Metabolism in Mice.Cell Mol Gastroenterol Hepatol. 2016 Dec 10;3(2):261-271. doi: 10.1016/j.jcmgh.2016.11.006. eCollection 2017 Mar. Cell Mol Gastroenterol Hepatol. 2016. PMID: 28275692 Free PMC article.
-
An Immune Gene Expression Signature Associated With Development of Human Hepatocellular Carcinoma Identifies Mice That Respond to Chemopreventive Agents.Gastroenterology. 2019 Nov;157(5):1383-1397.e11. doi: 10.1053/j.gastro.2019.07.028. Epub 2019 Jul 22. Gastroenterology. 2019. PMID: 31344396 Free PMC article.
-
iNKT cells prevent obesity-induced hepatic steatosis in mice in a C-C chemokine receptor 7-dependent manner.Int J Obes (Lond). 2018 Feb;42(2):270-279. doi: 10.1038/ijo.2017.200. Epub 2017 Aug 16. Int J Obes (Lond). 2018. PMID: 28811651 Free PMC article.
References
-
- Navaneethan U., Shen B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1598–1619. - PubMed
-
- Adams D.H., Eksteen B., Curbishley S.M. Immunology of the gut and liver: a love/hate relationship. Gut. 2008;57:838–848. - PubMed
-
- Grant A.J., Lalor P.F., Hubscher S.G. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–1072. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
