The Regulation of Steroid Action by Sulfation and Desulfation
- PMID: 26213785
- PMCID: PMC4591525
- DOI: 10.1210/er.2015-1036
The Regulation of Steroid Action by Sulfation and Desulfation
Abstract
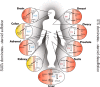
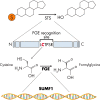
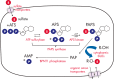
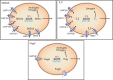
Steroid sulfation and desulfation are fundamental pathways vital for a functional vertebrate endocrine system. After biosynthesis, hydrophobic steroids are sulfated to expedite circulatory transit. Target cells express transmembrane organic anion-transporting polypeptides that facilitate cellular uptake of sulfated steroids. Once intracellular, sulfatases hydrolyze these steroid sulfate esters to their unconjugated, and usually active, forms. Because most steroids can be sulfated, including cholesterol, pregnenolone, dehydroepiandrosterone, and estrone, understanding the function, tissue distribution, and regulation of sulfation and desulfation processes provides significant insights into normal endocrine function. Not surprisingly, dysregulation of these pathways is associated with numerous pathologies, including steroid-dependent cancers, polycystic ovary syndrome, and X-linked ichthyosis. Here we provide a comprehensive examination of our current knowledge of endocrine-related sulfation and desulfation pathways. We describe the interplay between sulfatases and sulfotransferases, showing how their expression and regulation influences steroid action. Furthermore, we address the role that organic anion-transporting polypeptides play in regulating intracellular steroid concentrations and how their expression patterns influence many pathologies, especially cancer. Finally, the recent advances in pharmacologically targeting steroidogenic pathways will be examined.
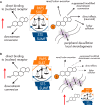
Figures
References
-
- Klyne W, Schachter B, Martin GF. The steroids of pregnant mares' urine; a method for the extraction of steroid sulphates and the isolation of allopregn-16-en-3β-ol-20-one sulphate. Biochem J. 1948;43:231–234. - PubMed
-
- Lipmann F. Biological sulfate activation and transfer. Science. 1958;128:575–580. - PubMed
-
- Klyne W. The C/D ring union in oestrone and equilenin. Nature. 1948;161:434. - PubMed
-
- Lewbart ML, Schneider JJ. Enzymatic synthesis of steroid sulfates. J Biol Chem. 1956;222:787–794. - PubMed
-
- Taylor AE, Keevil B, Huhtaniemi IT. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol. 2015;173:D1–D12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

