Photodynamic Treatment of Tumor with Bacteria Expressing KillerRed
- PMID: 26213989
- PMCID: PMC4516238
- DOI: 10.1371/journal.pone.0131518
Photodynamic Treatment of Tumor with Bacteria Expressing KillerRed
Abstract
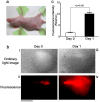
Photodynamic therapy (PDT) is a cancer treatment modality in which a photosensitizing dye is administered and exposed to light to kill tumor cells via the production of reactive oxygen species (ROS). A fundamental obstacle for PDT is the low specificity for staining solid tumors with dyes. Recently, a tumor targeting system guided by anaerobic bacteria was proposed for tumor imaging and treatment. Here, we explore the feasibility of the genetically encoded photosensitizer KillerRed, which is expressed in Escherichia coli, to treat tumors. Using nitroblue tetrazolium (NBT), we detected a lengthy ROS diffusion from the bodies of KillerRed-expressing bacteria in vitro, which demonstrated the feasibility of using bacteria to eradicate cells in their surroundings. In nude mice, Escherichia coli (E. coli) expressing KillerRed (KR-E. coli) were subcutaneously injected into xenografts comprising CNE2 cells, a human nasopharyngeal carcinoma cell line, and HeLa cells, a human cervical carcinoma cell line. KR-E. coli seemed to proliferate rapidly in the tumors as observed under an imaging system. When the intensity of fluorescence increased and the fluorescent area became as large as the tumor one day after KR-E. coli injection, the KR-E. coli-bearing tumor was irradiated with an orange light (λ = 540-580 nm). In all cases, the tumors became necrotic the next day and were completely eliminated in a few days. No necrosis was observed after the irradiation of tumors injected with a vehicle solution or a vehicle carrying the E. coli without KillerRed. In successfully treated mice, no tumor recurrence was observed for more than two months. E. coli genetically engineered for KillerRed expression are highly promising for the diagnosis and treatment of tumors when the use of bacteria in patients is cleared for infection safety.
Conflict of interest statement
Figures




References
-
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003; 3: 380–387. - PubMed
-
- Brown SB, Brown EA and Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004; 5: 497–508. - PubMed
-
- Chen J, Keltner L, Christophersen J, Zheng F, Krouse M, Singhal A, et al. New technology for deep light distribution in tissue for phototherapy. Cancer J. 2002; 8: 154–163. - PubMed
-
- Ethan DS, David D. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron. 1998; 54: 4151–4202.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

