Impact of C24:0 on actin-microtubule interaction in human neuronal SK-N-BE cells: evaluation by FRET confocal spectral imaging microscopy after dual staining with rhodamine-phalloidin and tubulin tracker green
- PMID: 26214025
- PMCID: PMC4520670
Impact of C24:0 on actin-microtubule interaction in human neuronal SK-N-BE cells: evaluation by FRET confocal spectral imaging microscopy after dual staining with rhodamine-phalloidin and tubulin tracker green
Abstract
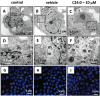
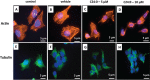
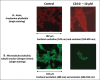
Disorganization of the cytoskeleton of neurons has major consequences on the transport of neurotransmitters via the microtubule network. The interaction of cytoskeleton proteins (actin and tubulin) was studied in neuronal SK-N-BE cells treated with tetracosanoic acid (C24:0), which is cytotoxic and increased in Alzheimer's disease patients. When SK-N-BE cells were treated with C24:0, mitochondrial dysfunctions and a non-apoptotic mode of cell death were observed. Fluorescence microscopy revealed shrunken cells with perinuclear condensation of actin and tubulin. Impact of C24:0 on actin-microtubule interaction in human neuronal SK-N-BE cells: evaluation by FRET confocal spectral imaging microscopy after dual staining with rhodamine-phalloidin and tubulin tracker green After staining with rhodamine-phalloidin and with an antibody raised against α-/β-tubulin, modifications of F-actin and α-/β-tubulin levels were detected by flow cytometry. Lower levels of α-tubulin were found by Western blotting. In C24:0-treated cells, spectral analysis and fluorescence recovery after photobleaching (FRAP) measured by confocal microscopy proved the existence of fluorescence resonance energy transfer (FRET) when actin and tubulin were stained with tubulin tracker and rhodamine-phalloidin demonstrating actin and tubulin co-localization/interaction. In control cells, no FRET was observed. Our data demonstrate quantitative changes in actin and tubulin, and modified interactions between actin and tubulin in SK-N-BE cells treated with C24:0. They also show that FRET confocal imaging microscopy is an interesting method for specifying the impact of cytotoxic compounds on cytoskeleton proteins.
Figures



Similar articles
-
Labeling cytoskeletal F-actin with rhodamine phalloidin or fluorescein phalloidin for imaging.Cold Spring Harb Protoc. 2010 May;2010(5):pdb.prot4947. doi: 10.1101/pdb.prot4947. Cold Spring Harb Protoc. 2010. PMID: 20439405
-
The role of actin, actomyosin and microtubules in defining cell shape during the differentiation of Naegleria amebae into flagellates.Eur J Cell Biol. 2007 Feb;86(2):85-98. doi: 10.1016/j.ejcb.2006.10.003. Epub 2006 Dec 26. Eur J Cell Biol. 2007. PMID: 17189659
-
A comparison of techniques for localizing actin and tubulin in hyphae of Saprolegnia ferax.J Histochem Cytochem. 1994 Apr;42(4):523-30. doi: 10.1177/42.4.7510320. J Histochem Cytochem. 1994. PMID: 7510320
-
Actin in emerging neurites is recruited from a monomer pool.Mol Neurobiol. 1992 Summer-Fall;6(2-3):95-106. doi: 10.1007/BF02780546. Mol Neurobiol. 1992. PMID: 1476678 Review.
-
Visualising the actin cytoskeleton.Microsc Res Tech. 1999 Oct 1;47(1):3-17. doi: 10.1002/(SICI)1097-0029(19991001)47:1<3::AID-JEMT2>3.0.CO;2-2. Microsc Res Tech. 1999. PMID: 10506758 Review.
Cited by
-
Interrogating Synaptic Architecture: Approaches for Labeling Organelles and Cytoskeleton Components.Front Synaptic Neurosci. 2019 Aug 23;11:23. doi: 10.3389/fnsyn.2019.00023. eCollection 2019. Front Synaptic Neurosci. 2019. PMID: 31507402 Free PMC article. Review.
-
Identification and evaluation of reference genes in the Chinese white wax scale insect Ericerus pela.Springerplus. 2016 Jun 21;5(1):791. doi: 10.1186/s40064-016-2548-z. eCollection 2016. Springerplus. 2016. PMID: 27390632 Free PMC article.
References
-
- Antonenkov VD, Grunau S, Ohlmeier S, et al. Peroxisomes are oxidative organelles. Antioxid Redox Signal. 2010;13:525–537. - PubMed
-
- Baarine M, Ragot K, Athias A, et al. Incidence of Abcd1 level on the induction of cell death and organelle dysfunctions triggered by very long chain fatty acids and TNF-α on oligodendrocytes and astrocytes. Neurotoxicology. 2012;33:212–228. - PubMed
-
- Belloc JF, Vincendeau P, Freyburger G, et al. Flow cytometric study of the activation of polymorphonuclear cells. J Leukoc Biol. 1990;48:353–358. - PubMed
-
- Benzecri JP. L’analyse des données, Tome 2: L’analyse des correspondances. Paris: Dunod; 1973.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
