Selenium supplementation for critically ill adults
- PMID: 26214143
- PMCID: PMC6517228
- DOI: 10.1002/14651858.CD003703.pub3
Selenium supplementation for critically ill adults
Abstract
Background: Selenium is a trace mineral essential to health and has an important role in immunity, defence against tissue damage and thyroid function. Improving selenium status could help protect against overwhelming tissue damage and infection in critically ill adults. This Cochrane review was originally published in 2004 updated in 2007 and again 2015.
Objectives: The primary objective was to examine the effect of nutrition supplemented with selenium or ebselen on mortality in critically ill patients.The secondary objective was to examine the relationship between selenium or ebselen supplementation and number of infections, duration of mechanical ventilation, length of intensive care unit stay and length of hospital stay.
Search methods: In this update, we searched the current issue of the Cochrane Central Register of Controlled Trials, the Cochrane Library (2014, Issue 5); MEDLINE (Ovid SP, to May 20, 2014), EMBASE (Ovid SP, to May 20, 2014), CAB, BIOSIS and CINAHL. We handsearched the reference lists of the newest reviews and cross-checked with our search in MEDLINE. We contacted the main authors of included studies to request any missed, unreported or ongoing studies. The latest search was performed up to 21 May 2014. The search is now from inception until 21 May 2014.
Selection criteria: We included randomized controlled trials (RCTs) irrespective of publication status, date of publication, blinding status, outcomes published or language. We contacted the trial investigators and authors in order to retrieve relevant and missing data.
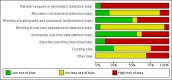
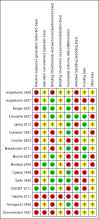
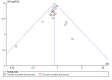
Data collection and analysis: Two review authors independently extracted data and we resolved any disagreements by discussion. Our primary outcome measure was all-cause mortality. We performed several subgroup and sensitivity analyses to assess the effects of selenium in critically ill patients. We presented pooled estimates of the effects of intervention as risk ratios (RRs) with 95% confidence intervals (CIs). We assessed the risk of bias through assessment of trial methodological components and the risk of random error through trial sequential analysis.
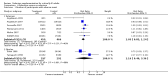
Main results: We included six new RCTs in this review update. In total we included 16 RCTs (2084 participants) in this review. Most trials were at high risk of bias. The availability of outcome data was limited and trials involving selenium supplementation were, with the exception of one trial, small regarding sample size. Thus the results must be interpreted with caution.Thirteen trials of intravenous sodium selenite showed a statistically significant reduction in overall mortality (RR 0.82, 95% CI 0.72 to 0.93, 1391 participants, very low quality of evidence). However, the overall point estimate on mortality is primarily influenced by trials of high risk of bias. Meta-analysis of three trials of ebselen had a RR of 0.83 (95% CI 0.52 to 1.34, 693 participants, very low quality of evidence).Nine trials of intravenous sodium selenite were analysed for 28 days mortality with no statistically significant difference (RR 0.84, 95% CI 0.69 to 1.02, 1180 participants, very low quality of evidence) while three trials were analysed for 90 days mortality with similar findings (RR 0.96, 95% Cl 0.78 to 1.18, 614 participants, very low quality of evidence).Two trials of ebselen were analysed for 90 days mortality and were not found to yield any benefit (RR 0.72, 95% Cl 0.42 to 1.22, 588 participants, very low quality of evidence).For mortality among intensive care patients selenium supplementation failed to indicate any statistically significant advantage (RR 0.88, 95% CI 0.77 to 1.01, nine trials, 1168 participants, very low quality of evidence).Six trials of intravenous sodium selenite found no statistically significant difference for participants developing infection (RR 0.96, 95% CI 0.75 to 1.23, 934 patients, very low quality of evidence). Similarly, three trials of ebselen provided data for participants developing infections (pyrexia, respiratory infections or meningitis) with no obvious benefit (RR 0.60, 95% CI 0.36 to 1.02, 685 participants, very low quality of evidence).Our analyses showed no effect of selenium or ebselen on adverse events (Selenium: RR 1.03, 95% Cl 0.85 to 1.24; six trials, 925 participants ; Ebselen: RR 1.16, 95% CI 0.40 to 3.36; two trials, 588 participants, very low quality of evidence).No clear evidence emerged in favour of selenium supplementation for outcomes such as number of days on a ventilator (mean difference (MD) -0.86, 95% CI -4.39 to 2.67, four trials, 191 participants, very low quality of evidence), length of intensive care unit stay (MD 0.54, 95% CI -2.27 to 3.34, seven trials, 934 participants, very low quality of evidence) or length of hospital stay (MD -3.33, 95% Cl -5.22 to -1.44, five trials, 693 participants, very low quality of evidence).The quality of trial methodology was low. Due to high risk of bias in the included trials, results must be interpreted with caution.
Authors' conclusions: Despite publication of a number of trials, the current evidence to recommend supplementation of critically ill patients with selenium or ebselen remains disputed. Trials are required which overcome the methodological inadequacies of the reviewed studies, particularly in relation to sample size, design and outcomes.
Conflict of interest statement
Mikkel Allingstrup declares no conflicts of interest.
Arash Afshari declares no conflicts of interest.
Figures
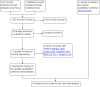









Update of
-
Selenium supplementation for critically ill adults.Cochrane Database Syst Rev. 2004 Oct 18;(4):CD003703. doi: 10.1002/14651858.CD003703.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2015 Jul 27;(7):CD003703. doi: 10.1002/14651858.CD003703.pub3. PMID: 15495061 Updated.
Similar articles
-
Selenium supplementation for critically ill adults.Cochrane Database Syst Rev. 2004 Oct 18;(4):CD003703. doi: 10.1002/14651858.CD003703.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2015 Jul 27;(7):CD003703. doi: 10.1002/14651858.CD003703.pub3. PMID: 15495061 Updated.
-
Antithrombin III for critically ill patients.Cochrane Database Syst Rev. 2016 Feb 8;2(2):CD005370. doi: 10.1002/14651858.CD005370.pub3. Cochrane Database Syst Rev. 2016. PMID: 26858174 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults.Cochrane Database Syst Rev. 2016 Jun 27;2016(6):CD002787. doi: 10.1002/14651858.CD002787.pub3. Cochrane Database Syst Rev. 2016. PMID: 27347773 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Identifying a target group for selenium supplementation in high-risk cardiac surgery: a secondary analysis of the SUSTAIN CSX trial.Intensive Care Med Exp. 2023 Dec 8;11(1):89. doi: 10.1186/s40635-023-00574-8. Intensive Care Med Exp. 2023. PMID: 38063975 Free PMC article.
-
Clinical Significance of Micronutrient Supplementation in Critically Ill COVID-19 Patients with Severe ARDS.Nutrients. 2021 Jun 20;13(6):2113. doi: 10.3390/nu13062113. Nutrients. 2021. PMID: 34203015 Free PMC article.
-
Zero-, one-, two- and three-dimensional supramolecular architectures sustained by Se…O chalcogen bonding: A crystallographic survey.Coord Chem Rev. 2021 Jan 15;427:213586. doi: 10.1016/j.ccr.2020.213586. Epub 2020 Oct 17. Coord Chem Rev. 2021. PMID: 33100367 Free PMC article. Review.
-
The influence of early selenium supplementation on trauma patients: A propensity-matched analysis.Front Nutr. 2022 Dec 8;9:1062667. doi: 10.3389/fnut.2022.1062667. eCollection 2022. Front Nutr. 2022. PMID: 36570123 Free PMC article.
-
Oxidative Stress and Neonatal Respiratory Extracorporeal Membrane Oxygenation.Front Physiol. 2018 Dec 4;9:1739. doi: 10.3389/fphys.2018.01739. eCollection 2018. Front Physiol. 2018. PMID: 30564143 Free PMC article. Review.
References
References to studies included in this review
Angstwurm 1999 {published data only}
-
- Angstwurm MWA, Schopohl J, Gaertner R. Selenium substitution has no direct effect on thyroid hormone metabolism in critically ill patients. European Journal of Endocrinology 2004;151(1):47‐54. [MEDLINE: ] - PubMed
-
- Angstwurm MWA, Schottdorf J, Schopohl J, Gaertner R. Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Critical Care Medicine 1999;27(9):1807‐13. [MEDLINE: ] - PubMed
-
- Gärtner R, Angstwurm MWA, Schopohl J. Selenium replacement in patients with SIRS improves clinical outcome. Clinical Nutrition 2002;21(Suppl 1):66.
-
- Gärtner R, Angstwurm MWA, Schottdorf J. Selenium administration in sepsis patients. Medizinische Klinik 1997;92(Suppl 3):12‐4. [PUBMED: 9417486] - PubMed
Angstwurm 2007 {published data only}
-
- Angstwurm MW, Englemann L, Zimmermann T, Lehmann C, Spes CH, Abel P, et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo‐controlled, multiple‐center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Critical Care Medicine 2007;35(1):118‐26. - PubMed
-
- Angstwurm MWA, Gaertner R. Selenium substitution in severe sepsis as useful additive? Preliminary results of the SIC study. Intensive Care Medicine 2005;31(Suppl 1):S84.
-
- Forceville X. The need for precise dose information of sodium selenite in the SIC study and rectification of GPx‐3 plasma concentration. Critical Care Medicine 2008;36(2):656‐7. - PubMed
-
- Forceville X. The need for precise dose information of sodium selenite in the SIC study and rectification of GPx‐3 plasmaconcentration. Erratum. Critical Care Medicine 2008;36(7):2224. - PubMed
-
- Gaertner R. Sodium selenite as an adjunctive therapy for sepsis ‐ clinical data from a multi‐centre intervention study (SIC Study). DIVI Congress, Hamburg, Symposium 'Adjunctive therapy in sepsis: what has been confirmed?'. 4 December 2004.
Berger 2001 {published data only}
-
- Berger MM, Baines M, Chioléro RL, Wardle CA, Cayeux C, Shenkin A. Influence of early trace element and vitamin E supplements on antioxidant status after major trauma: a controlled trial. Nutrition Research 2001;21:41‐54.
-
- Berger MM, Reymond MJ, Shenkin A, Rey F, Wardle C, Cayeux C, et al. Influence of selenium supplements on the post‐traumatic alterations of the thyroid axis: a placebo‐controlled trial. Intensive Care Medicine 2001;27(1):91‐100. [MEDLINE: ] - PubMed
Forceville 2007 {published data only}
-
- Forceville X, Bellissant E. Selenium, as sodium selenite, in the treatment of septic shock. http://clinicaltrials.gov/show/NCT00207844 (accessed 8 August 2006).
Janka 2013 {published data only}
Kuklinski 1991 {published data only}
-
- Kuklinski B, Buchner M, Schweder R, Nagel R. Acute pancreatitis ‐ a "free radical disease". Decrease of lethality by sodium selenite (Na2SeO3) therapy [Akute pankreatitis ‐ eine "free radical disease". Letalitatssenkung durch natriumselenit (Na2SeO3)‐therapie]. Zeitschrift für die Gesamte Innere Medizin und Ihre Grenzgebiete 1991;46(5):145‐9. [MEDLINE: ] - PubMed
Lindner 2004 {published data only}
-
- Lindner D, Lindner J, Baumann G, Dawczynski H, Bauch K. Investigation of antioxidant therapy with sodium selenite in acute pancreatitis. A prospective randomized blind trial [Untersuchung zur antioxidativen therapie mit natriumselenit bei akuter pankreatitis. Eine prospektive, randomisierte blindstudie]. Medizinische Klinik 2004;99(12):708‐12. [MEDLINE: ] - PubMed
Manzanares 2011 {published data only}
-
- Manzanares W, Biestro A, Torre MH, Galusso F, Fachin G, Hardy G. High‐dose selenium reduces ventilator‐associated pneumonia and illness severity in critically ill patients with systemic inflammation. Intensive Care Medicine 2011;37(7):1120‐7. - PubMed
Mishra 2007 {published data only}
-
- Mishra V, Baines M, Perry S, McLaughlin J, Carson J, Wenstone R, et al. Selenium supplementation and outcome in septic ICU patients. Clinica Chimica Acta 2005;355(Suppl 1):S45.
-
- Mishra V, Baines M, Perry SE, McLaughlin PJ, Carson J, Wenstone R, et al. Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clinical Nutrition 2007;26(1):41‐50. - PubMed
-
- Mishra V, Perry S, Baines M, McLaughlin J, Carson J, Wenstone R, et al. Selenium replacement, HLA‐DR expression and outcome in septic ICU patients. Intensive Care Medicine 2004;30(Suppl 1):110.
Montoya 2009 {published data only}
-
- Montoya CG, Luna AH, Silva JAV, Guzmán CO, Sánchez JA, Granillo JF. High doses of selenium in severe sepsis [Efecto antiinflamatorio del selenio en pacientes sépticos]. Revista de la Asociación Mexicana de Medicina Crítica y Terapia Intensiva 2009;23(4):199‐205.
Ogawa 1999 {published data only}
-
- Ogawa A, Yoshimoto T, Kikuchi H, Sano K, Saito I, Yamaguchi T, et al for the Ebselen Study Group. Ebselen in acute middle cerebral artery occlusion: a placebo‐controlled, double‐blind clinical trial. Cerebrovascular Diseases 1999;9(2):112‐8. [MEDLINE: ] - PubMed
Saito 1998 {published data only}
-
- Asano T, Takakura K, Sano K. Comparative analysis of the clinical effects of water‐soluble (AVS) and ‐insoluble (ebselen) antioxidants on the delayed ischemic neurological deficits after aneurysmal subarachnoid hemorrhage. Journal of Stroke and Cerebrovascular Diseases 1996;6(Suppl 1):188‐92.
-
- Saito I, Abe H, Yoshimoto T, Asano T, Takakura K, Ohta T, et al. Multicentre randomised clinical trial of ebselen with aneurysmal subarachnoid hemorrhage. Journal of Cerebral Blood Flow and Metabolism 1995;15(Suppl 1):162.
-
- Saito I, Asano T, Sano K, Takakura K, Abe H, Yoshimoto T, et al. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery 1998;42(2):269‐78. [MEDLINE: ] - PubMed
SIGNET 2011 {published and unpublished data}
-
- Andrews PJ. Randomised trial of glutamine and selenium supplemented parenteral nutrition (PN) for critically ill patients. http://www.isrctn.com/ISRCTN87144826 (accessed 21 October 2014).
-
- Andrews PJ, Avenell A, Noble DW, Campbell MK, Battison CG, Croal BL, et al. Randomised trial of glutamine and selenium supplemented parenteral nutrition for critically ill patients. Protocol Version 9, 19 February 2007 known as SIGNET (Scottish Intensive care Glutamine or seleNium Evaluative Trial. Trials 2007;8:25. - PMC - PubMed
-
- Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011;342:d1542. - PubMed
-
- Andrews PJ, Avenell A, and the SIGNET Trial Group. Results of The SIGNET Trial ‐ a randomised controlled trial of glutamine and/or selenium supplemented parenteral nutrition in critical illness. Proceedings of the Nutrition Society 2010;69(OCE2):E170.
-
- Andrews PJ, Simpson WG and the SIGNET Trial group. Results of the SIGNET Trial ‐ a randomised controlled trial of glutamine and/or selenium supplemented parenteral nutrition in critical illness. Clinical Nutrition Supplements 2010;5(2):22.
Valenta 2011 {published data only}
-
- Brodska H, Kazda A, Valenta J, Hendl J. Effect of high‐dose selenium substitution on selected laboratory parameters and prognosis in critically ill patients. Critical Care 2009;13(Suppl 1):S62.
-
- Brodska H, Kazda A, Valenta J, Stach Z, Hendl J, Pelinkova K. High dose of selenium in critically ill. Clinical Nutrition Supplements 2007;2(2):20‐1.
-
- Brodska H, Kazda A, Valenta J, Stach Z, Hendl J, Pelinkova K, et al. Laboratory monitoring during high dose selenium substitution in critically ill patients. Clinical Chemistry and Laboratory Medicine 2007;45:S396.
-
- Brodska H, Kohout P, Malickova K, Kazda A, Valenta J. Influence of high selenium doses supplementation on markers of nutritional status. Clinical Nutrition Supplements 2010;5(2):115.
-
- Kazda A, Brodska H, Valenta J, Vinglerova M, Hendl J, Stach Z, et al. Selenium and its substitution in critically ill patients. Critical Care 2006;10(Suppl 1):S79‐80.
Yamaguchi 1998 {published data only}
-
- Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, et al for the Ebselen Study Group. Ebselen in acute ischemic stroke: a placebo‐controlled, double‐blind clinical trial. Stroke 1998;29(1):12‐7. [MEDLINE: ] - PubMed
Zimmermann 1997 {published data only}
-
- Zimmermann T, Albrecht S, Kühne H, Vogelsang U, Grützmann R, Kopprasch S. Selenium administration in patients with sepsis syndrome. A prospective randomized study [Selensubstitution bei sepsispatienten. Eine prospektiv randomisierte studie]. Medizinische Klinik 1997;92(Suppl 3):3‐4. [MEDLINE: ] - PubMed
-
- Zimmermann T, Albrecht S, Gagern G. Molecular‐biology studies of a multicenter phase III study (SIC‐study) [Molekularbiologische untersuchungen zur multizentrischen phase‐III‐studie (SIC‐studie)]. Medizinische Klinik 1999;94(Suppl 3):58‐61. - PubMed
References to studies excluded from this review
Berger 1998 {published data only}
-
- Berger MM, Spertini F, Shenkin A, Wardle C, Wiesner L, Schindler C, et al. Trace element supplementation modulates pulmonary infection rates after major burns: a double‐blind, placebo‐controlled trial. American Journal of Clinical Nutrition 1998;68(2):365‐71. [MEDLINE: ] - PubMed
Berger 2004a {published data only}
-
- Berger M, Binnert C, Baines M, Raffoul W, Cayeux M, Chiolero R, et al. Trace element supplements influence protein metabolism and tissue levels after major burns. Intensive Care Medicine 2004;30(Suppl 1):61.
Berger 2004b {published data only}
-
- Berger M, Soguel L, Schindler C, Revelly J, Chiolero R. Antioxidant micronutrients after complex cardiac surgery and clinical evolution? Preliminary data. Intensive Care Medicine 2004;30(Suppl 1):175.
Berger 2005a {published data only}
-
- Berger MM, Eggimann P, Revelly JP, Raffoul W, Shenkin A, Chiolero R. Trace element supplements are associated with fewer nosocomial pneumonia after major burns. Intensive Care Medicine 2005;31(Suppl 1):77.
-
- Berger MM, Eggimann P, Revelly JP, Raffoul W, Shenkin A, Chiolero RL. Selenium supplements reduce the incidence of nosocomial pneumonia after major burns. Clinical Nutrition 2005;24:614.
Berger 2005b {published data only}
-
- Berger MM, Soguel L, Pinget C, Revelly JP, Schindler C, Chiolero RL. Antioxidant supplements modulate clinical course after complex cardiac surgery, and major trauma. Intensive Care Medicine 2005;31(Suppl 1):32.
-
- Berger MM, Soguel L, Pinget C, Revelly JP, Schindler C, Chiolero RL. Antioxidant supplements modulate clinical course after complex cardiac surgery, major trauma, and subarachnoid haemorrhage. Clinical Nutrition 2005;24(4):616.
Berger 2008 {published data only}
-
- Berger M, Soguel L, Shenkin A, RevellyJP, Pinget C, Baines M, et al. Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Critical Care 2008;12(4):R101. [DOI: 10.1186/cc6981] - DOI - PMC - PubMed
Börner 1997 {published data only}
-
- Börner J, Zimmermann T, Albrecht S, Roesner D. Selenium administration in severe inflammatory surgical diseases and burns in childhood [Selensubstitution bei schweren entzündlichen chirurgischen krankheitsbildern sowie verbrennungen un verbrühungen in kindesalter]. Medizinische Klinik 1997;92(Suppl 3):17‐9. [MEDLINE: ] - PubMed
Collier 2008 {published data only}
Kiessling 2006 {published data only}
-
- Kiessling AH, Isgro F, Skuras JA, Kammerer I, Lehmann A, Saggau W. Selenium application in intensive care medicine. Intensive Care Medicine 2006;32(Suppl 1):89.
Porter 1999 {published data only}
-
- Porter JM, Ivatury RR, Azimuddin K, Swami R. Antioxidant therapy in the prevention of organ dysfunction syndrome and infectious complications after trauma: early results of a prospective randomized study. American Surgeon 1999;65(5):478‐83 (Erratum in American Surgeon 1999;65:902). [MEDLINE: ] - PubMed
REDOXS 2011 {published data only}
Stoppe 2010 {published data only}
-
- Stoppe C, Wildenhues A, Metzelder S, Schalte G, Menon AK, Rossaint R, et al. The effects of high dose selenium supplementation on perioperative systemic inflammation in patients undergoing open heart surgery. Intensive Care Medicine 2010;36(Suppl 2):S316.
Thiele 1997 {published data only}
-
- Thiele R, Wagner D, Gassel M, Winnefeld K, Pleissner J, Pfeifer R. Selenium substitution in acute myocardial infarct [Selensubstitution bei akutem myokardinfarkt]. Medizinische Klinik 1997;92(Suppl 3):26‐8. [MEDLINE: ] - PubMed
Uden 1990 {published data only}
-
- Uden S, Bilton D, Nathan L, Hunt LP, Main C, Braganza JM. Antioxidant therapy for recurrent pancreatitis: placebo‐controlled trial. Alimentary Pharmacology and Therapeutics 1990;4(4):357‐71. [MEDLINE: ] - PubMed
Watters 2002 {published data only}
-
- Watters JM, Vallerand A, Kirkpatrick SM, Abbott HE, Norris S, Wells G, et al. Limited effects of micronutrient supplementation on strength and physical function after abdominal aortic aneursymectomy. Clinical Nutrition 2002;21(4):321‐7. [MEDLINE: ] - PubMed
Wollschläger 1997 {published data only}
-
- Wollschläger S, Ludwig K, Meissner D, Porst H. Effect of selenium administration on various laboratory parameters in patients with acute pancreatitis [Einfluß einer selensubstitution auf verschiedene laborparameter bei patienten mit akuter pankreatitis]. Medizinische Klinik 1997;92(Suppl 3):22‐4. [MEDLINE: ] - PubMed
References to studies awaiting assessment
Geoghegan 2009 {published data only}
-
- Geoghegan M, Eaton S, Mandersloot G, Healy M, McAuley DF, Rayman MP, et al. Selenium decreases lipid peroxidation in critically‐ill patients with sub‐arachnoid haemorrhage. Clinical Nutrition Supplements 2009;4(2):31.
Yamaguchi 2003 {published data only}
-
- Yamaguchi T, for Ebselen Study Group. Phase III trial of Ebselen (abstract). American Stroke Association 28th International Stroke Conference, 2003 February 13 ‐15; Phoenix, Arizona. 2003.
References to ongoing studies
SEREAL 2012 {published data only}
-
- Selenium Replacement and Serum Selenium Level in Severe Sepsis and Septic Shock Patients. Ongoing study September 2012.
SISPCT {published data only}
-
- Reinhart K. Placebo controlled trial of sodium selenite and procalcitonin guided antimicrobial therapy in severe sepsis. http://clinicaltrials.gov/ct2/show/NCT00832039. [NCT00832039]
Additional references
Angstwurm 2006
-
- Angstwurm MW, Gaertner R. Practicalities of selenium supplementation in critically ill patients. Current Opinion in Clinical Nutrition and Metabolic Care 2006;9(3):233‐8. - PubMed
ASPEN
-
- American Society for Parenteral and Enteral Nutrition. https://www.nutritioncare.org/ (accessed 21 October 2014).
Berger 2007
-
- Berger M, Baines M, Raffoul W, Benathan M, Chiolero R, Reeves C. Trace element supplementation after major burns modulates antioxidant status and clinical course by way of increased tissue trace element concentrations.. The American journal of clinical nutrition 2007;85:1293‐300. [PUBMED: 17490965] - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [MEDLINE: ] - PubMed
Bulger 2001
-
- Bulger EM, Maier RV. Antioxidants in critical illness. Archives of Surgery 2001;136(10):1201‐7. [MEDLINE: ] - PubMed
Chan 2004
-
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [MEDLINE: ] - PubMed
ESPEN
-
- European Society for Clinical Nutrition and Metabolism. http://www.espen.org/ (Accessed 21 October 2014).
Geoghegan 2006
-
- Geoghegan M, McAuley D, Eaton, Powell‐Tuck J. Selenium in critical illness. Current Opinion in Critical Care 2006;12(2):136‐41. - PubMed
Giladi 2011
-
- Giladi A, Dossett L, Fleming S, Abumrad N, Cotton B. High‐dose antioxidant administration is associated with a reduction in post‐injury complications in critically ill trauma patients. Injury 2011;42:78‐82. [PUBMED: 20149369] - PubMed
Heyland 2005
-
- Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Mediicine 2005;31(3):327‐37. [MEDLINE: ] - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539–58. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [DOI: 10.1002/9780470712184] - DOI
Hozo 2005
Huang 2013
Hutton 2000
-
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Journal of the Royal Statistical Society Series 2000;49:359‐70. [MEDLINE: ]
Jennett 1975
-
- Jennett B, Bond M. Assessment of outcome after severe brain damage. A practical scale. Lancet 1975;1:480‐4. [MEDLINE: ] - PubMed
Lan 1983
-
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659‐63.
Landucci 2014
Manzanares 2009
-
- Manzanares W, Hardy G. Selenium supplementation in the critically ill: posology and pharmacokinetics. Current Opinion in Clinical Nutrition and Metabolic Care 2009;12(3):273‐80. - PubMed
Manzanares 2012
Moher 2010
-
- Moher D, Hopewell S, Schulz K, Montori V, Gøtzsche P, Devereaux P, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials.. International journal of surgery 2010;10:28‐55. [PUBMED: 22036893] - PubMed
Parnham 2000
-
- Parnham M, Sies H. Ebselen: prospective therapy for cerebral ischaemia. Expert Opinion on Investigational Drugs 2000;9(3):607‐19. [MEDLINE: ] - PubMed
Pogue 1997
-
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: Utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [MEDLINE: ] - PubMed
Rayman 2012
-
- Rayman M. Selenium and human health. Lancet 2012;379:1256‐68. [PUBMED: 22381456] - PubMed
RevMan 5.3 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sattar 1997
-
- Sattar N, Eatock F, Fell GS, O'Reilly D. Selenium: an acute‐phase reactant?. Annals of Clinical Biochemistry 1997;34(Pt 4):437‐9. [MEDLINE: ] - PubMed
Shah 1989
-
- Shah S, Vanclay F, Cooper B. Predicting discharge status at commencement of stroke rehabilitation. Stroke 1989;20(6):766‐9. [MEDLINE: ] - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [MEDLINE: ] - PubMed
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). CTU (Copenhagen Trial Unit). 2011. http://www.ctu.dk/TSA/files/tsa_manual.pdf (accessed 21 Oct 2014):1‐118.
Trial Sequential Analysis (TSA) [Computer program]
-
- Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark. www.ctu.dk/tsa. TSA ‐ Trial Sequential Analysis. Copenhagen Trial Unit (CTU). Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark. www.ctu.dk/tsa, 2011.
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [MEDLINE: ] - PubMed
References to other published versions of this review
Avenell 2002
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

