Tunable and reversible drug control of protein production via a self-excising degron
- PMID: 26214256
- PMCID: PMC4543534
- DOI: 10.1038/nchembio.1869
Tunable and reversible drug control of protein production via a self-excising degron
Abstract
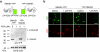
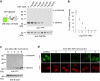
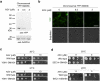
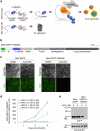
An effective method for direct chemical control over the production of specific proteins would be widely useful. We describe small molecule-assisted shutoff (SMASh), a technique in which proteins are fused to a degron that removes itself in the absence of drug, resulting in the production of an untagged protein. Clinically tested HCV protease inhibitors can then block degron removal, inducing rapid degradation of subsequently synthesized copies of the protein. SMASh allows reversible and dose-dependent shutoff of various proteins in multiple mammalian cell types and in yeast. We also used SMASh to confer drug responsiveness onto an RNA virus for which no licensed inhibitors exist. As SMASh does not require the permanent fusion of a large domain, it should be useful when control over protein production with minimal structural modification is desired. Furthermore, as SMASh involves only a single genetic modification and does not rely on modulating protein-protein interactions, it should be easy to generalize to multiple biological contexts.
Figures


Comment in
-
Methods: A small-molecule SMASh hit.Nat Chem Biol. 2015 Sep;11(9):637-8. doi: 10.1038/nchembio.1886. Nat Chem Biol. 2015. PMID: 26284670 Free PMC article. No abstract available.
-
Synthetic biology. Stop the presses.Nat Methods. 2015 Sep;12(9):811. doi: 10.1038/nmeth.3574. Nat Methods. 2015. PMID: 26554091 No abstract available.
References
-
- Huang CJ, et al. Conditional expression of a myocardium-specific transgene in zebrafish transgenic lines. Dev. Dyn. 2005;233:1294–1303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

