Dynamics and Membrane Interactions of Protein Kinase C
- PMID: 26214365
- PMCID: PMC4979571
- DOI: 10.1021/acs.biochem.5b00565
Dynamics and Membrane Interactions of Protein Kinase C
Abstract
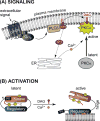
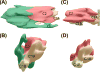
Protein kinase C (PKC) is a family of Ser/Thr kinases that regulate a multitude of cellular processes through participation in the phosphoinositide signaling pathway. Significant research efforts have been directed at understanding the structure, function, and regulatory modes of the enzyme since its discovery and identification as the first receptor for tumor-promoting phorbol esters. The activation of PKC involves a transition from the cytosolic autoinhibited latent form to the membrane-associated active form. The membrane recruitment step is accompanied by the conformational rearrangement of the enzyme, which relieves autoinhibitory interactions and thereby allows PKC to phosphorylate its targets. The multidomain structure and intrinsic flexibility of PKC present remarkable challenges and opportunities for the biophysical and structural biology studies of this class of enzymes and their interactions with membranes, the major focus of this Current Topic. I will highlight the recent advances in the field, outline the current challenges, and identify areas where biophysics and structural biology approaches can provide insight into the isoenzyme-specific regulation of PKC activity.
Figures

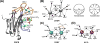


References
-
- Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. 2. Proenzyme and its actiavtion by calcium-dependent protease from rat brain. J Biol Chem. 1977;252:7610–7616. - PubMed
-
- Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. 1. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252:7603–7609. - PubMed
-
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct Activation of Calcium-Activated, Phospholipid-Dependent Protein-Kinase by Tumor-Promoting Phorbol Esters. J Biol Chem. 1982;257:7847–7851. - PubMed
-
- Newton AC. Protein kinase C: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. - PubMed
-
- Rosse C, Linch M, Kermorgant S, Cameron AJM, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nature Reviews Molecular Cell Biology. 2010;11:103–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

