Therapeutic cancer vaccines
- PMID: 26214521
- PMCID: PMC4588240
- DOI: 10.1172/JCI80009
Therapeutic cancer vaccines
Abstract
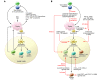
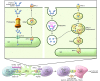
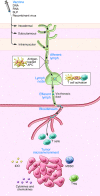
The clinical benefit of therapeutic cancer vaccines has been established. Whereas regression of lesions was shown for premalignant lesions caused by HPV, clinical benefit in cancer patients was mostly noted as prolonged survival. Suboptimal vaccine design and an immunosuppressive cancer microenvironment are the root causes of the lack of cancer eradication. Effective cancer vaccines deliver concentrated antigen to both HLA class I and II molecules of DCs, promoting both CD4 and CD8 T cell responses. Optimal vaccine platforms include DNA and RNA vaccines and synthetic long peptides. Antigens of choice include mutant sequences, selected cancer testis antigens, and viral antigens. Drugs or physical treatments can mitigate the immunosuppressive cancer microenvironment and include chemotherapeutics, radiation, indoleamine 2,3-dioxygenase (IDO) inhibitors, inhibitors of T cell checkpoints, agonists of selected TNF receptor family members, and inhibitors of undesirable cytokines. The specificity of therapeutic vaccination combined with such immunomodulation offers an attractive avenue for the development of future cancer therapies.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

