Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification
- PMID: 26214526
- PMCID: PMC4563750
- DOI: 10.1172/JCI80313
Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification
Erratum in
-
Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification.J Clin Invest. 2015 Sep;125(9):3723. doi: 10.1172/JCI84059. Epub 2015 Sep 1. J Clin Invest. 2015. PMID: 26325037 Free PMC article. No abstract available.
Abstract
Bone formation during fracture repair inevitably initiates within or around extravascular deposits of a fibrin-rich matrix. In addition to a central role in hemostasis, fibrin is thought to enhance bone repair by supporting inflammatory and mesenchymal progenitor egress into the zone of injury. However, given that a failure of efficient fibrin clearance can impede normal wound repair, the precise contribution of fibrin to bone fracture repair, whether supportive or detrimental, is unknown. Here, we employed mice with genetically and pharmacologically imposed deficits in the fibrin precursor fibrinogen and fibrin-degrading plasminogen to explore the hypothesis that fibrin is vital to the initiation of fracture repair, but impaired fibrin clearance results in derangements in bone fracture repair. In contrast to our hypothesis, fibrin was entirely dispensable for long-bone fracture repair, as healing fractures in fibrinogen-deficient mice were indistinguishable from those in control animals. However, failure to clear fibrin from the fracture site in plasminogen-deficient mice severely impaired fracture vascularization, precluded bone union, and resulted in robust heterotopic ossification. Pharmacological fibrinogen depletion in plasminogen-deficient animals restored a normal pattern of fracture repair and substantially limited heterotopic ossification. Fibrin is therefore not essential for fracture repair, but inefficient fibrinolysis decreases endochondral angiogenesis and ossification, thereby inhibiting fracture repair.
Figures






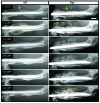


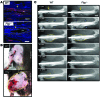
Similar articles
-
Fibrinolysis as a Target to Enhance Fracture Healing.N Engl J Med. 2015 Oct 29;373(18):1776-8. doi: 10.1056/NEJMcibr1510090. N Engl J Med. 2015. PMID: 26510027 Free PMC article. Review. No abstract available.
-
Profibrinolytic effects of metalloproteinases during skin wound healing in the absence of plasminogen.J Invest Dermatol. 2008 Aug;128(8):2092-101. doi: 10.1038/jid.2008.54. Epub 2008 Mar 13. J Invest Dermatol. 2008. PMID: 18337830
-
Plasminogen plays a crucial role in bone repair.J Bone Miner Res. 2013 Jul;28(7):1561-74. doi: 10.1002/jbmr.1921. J Bone Miner Res. 2013. PMID: 23456978
-
Healing of corneal epithelial defects in plasminogen- and fibrinogen-deficient mice.Invest Ophthalmol Vis Sci. 1998 Mar;39(3):502-8. Invest Ophthalmol Vis Sci. 1998. PMID: 9501859
-
Fibrinogen and fibrin.Annu Rev Biochem. 1984;53:195-229. doi: 10.1146/annurev.bi.53.070184.001211. Annu Rev Biochem. 1984. PMID: 6383194 Review. No abstract available.
Cited by
-
Plasminogen activation in the musculoskeletal acute phase response: Injury, repair, and disease.Res Pract Thromb Haemost. 2020 Jun 14;4(4):469-480. doi: 10.1002/rth2.12355. eCollection 2020 May. Res Pract Thromb Haemost. 2020. PMID: 32548548 Free PMC article. Review.
-
Variable Response to Antifibrinolytics Correlates with Blood-loss and Transfusion in Posterior Spinal Fusion.Spine Deform. 2022 Jul;10(4):841-851. doi: 10.1007/s43390-022-00489-6. Epub 2022 Mar 5. Spine Deform. 2022. PMID: 35247191 Free PMC article.
-
Novel preclinical murine model of trauma-induced elbow stiffness.J Exp Orthop. 2018 Sep 18;5(1):36. doi: 10.1186/s40634-018-0155-3. J Exp Orthop. 2018. PMID: 30229498 Free PMC article.
-
Plasminogen Regulates Fracture Repair by Promoting the Functions of Periosteal Mesenchymal Progenitors.J Bone Miner Res. 2021 Nov;36(11):2229-2242. doi: 10.1002/jbmr.4423. Epub 2021 Sep 8. J Bone Miner Res. 2021. PMID: 34378815 Free PMC article.
-
Lymphatic platelet thrombosis limits bone repair by precluding lymphatic transporting DAMPs.Nat Commun. 2025 Jan 18;16(1):829. doi: 10.1038/s41467-025-56147-8. Nat Commun. 2025. PMID: 39827193 Free PMC article.
References
-
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green’s Fractures In Adults. Philadelphia, Pennsylvania, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
-
- Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

