Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction
- PMID: 26214527
- PMCID: PMC4563759
- DOI: 10.1172/JCI81321
Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction
Abstract
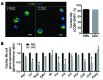
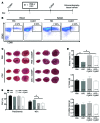
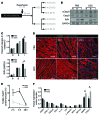
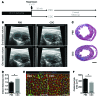
Ischemic injury in the heart induces an inflammatory cascade that both repairs damage and exacerbates scar tissue formation. Cardiosphere-derived cells (CDCs) are a stem-like population that is derived ex vivo from cardiac biopsies; they confer both cardioprotection and regeneration in acute myocardial infarction (MI). While the regenerative effects of CDCs in chronic settings have been studied extensively, little is known about how CDCs confer the cardioprotective process known as cellular postconditioning. Here, we used an in vivo rat model of ischemia/reperfusion (IR) injury-induced MI and in vitro coculture assays to investigate how CDCs protect stressed cardiomyocytes. Compared with control animals, animals that received CDCs 20 minutes after IR had reduced infarct size when measured at 48 hours. CDCs modified the myocardial leukocyte population after ischemic injury. Specifically, introduction of CDCs reduced the number of CD68+ macrophages, and these CDCs secreted factors that polarized macrophages toward a distinctive cardioprotective phenotype that was not M1 or M2. Systemic depletion of macrophages with clodronate abolished CDC-mediated cardioprotection. Using both in vitro coculture assays and a rat model of adoptive transfer after IR, we determined that CDC-conditioned macrophages attenuated cardiomyocyte apoptosis and reduced infarct size, thereby recapitulating the beneficial effects of CDC therapy. Together, our data indicate that CDCs limit acute injury by polarizing an effector macrophage population within the heart.
Figures






Comment in
-
Emerging roles for macrophages in cardiac injury: cytoprotection, repair, and regeneration.J Clin Invest. 2015 Aug 3;125(8):2927-30. doi: 10.1172/JCI83191. Epub 2015 Jul 27. J Clin Invest. 2015. PMID: 26214519 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

