Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas
- PMID: 26214590
- PMCID: PMC4916843
- DOI: 10.1038/ng.3361
Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas
Abstract
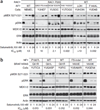
We report on whole-exome sequencing (WES) of 213 melanomas. Our analysis established NF1, encoding a negative regulator of RAS, as the third most frequently mutated gene in melanoma, after BRAF and NRAS. Inactivating NF1 mutations were present in 46% of melanomas expressing wild-type BRAF and RAS, occurred in older patients and showed a distinct pattern of co-mutation with other RASopathy genes, particularly RASA2. Functional studies showed that NF1 suppression led to increased RAS activation in most, but not all, melanoma cases. In addition, loss of NF1 did not predict sensitivity to MEK or ERK inhibitors. The rebound pathway, as seen by the induction of phosphorylated MEK, occurred in cells both sensitive and resistant to the studied drugs. We conclude that NF1 is a key tumor suppressor lost in melanomas, and that concurrent RASopathy gene mutations may enhance its role in melanomagenesis.
Figures



References
-
- Hammond D, et al. Melanoma-associated mutations in protein phosphatase 6 cause chromosome instability and DNA damage owing to dysregulated Aurora-A. J. Cell Sci. 2013;126:3429–3440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

