A Screen for Epigenetically Silenced microRNA Genes in Gastrointestinal Stromal Tumors
- PMID: 26214687
- PMCID: PMC4516245
- DOI: 10.1371/journal.pone.0133754
A Screen for Epigenetically Silenced microRNA Genes in Gastrointestinal Stromal Tumors
Abstract
Background: Dysregulation of microRNA (miRNA) has been implicated in gastrointestinal stromal tumors (GISTs) but the mechanism is not fully understood. In this study, we aimed to explore the involvement of epigenetic alteration of miRNA genes in GISTs.
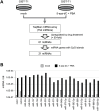
Methods: GIST-T1 cells were treated with 5-aza-2'-deoxycytidine (5-aza-dC) and 4-phenylbutyric acid (PBA), after which miRNA expression profiles were analyzed using TaqMan miRNA arrays. DNA methylation was then analyzed using bisulfite pyrosequencing. The functions of miRNAs were examined using MTT assays, wound-healing assays, Boyden chamber assays and Matrigel invasion assays. Gene expression microarrays were analyzed to assess effect of ectopic miRNA expression in GIST-T1 cells.
Results: Of the 754 miRNAs analyzed, 61 were significantly upregulated in GIST-T1 cells treated with 5-aza-dC plus PBA. Among those, 21 miRNA genes were associated with an upstream CpG island (CGI), and the CGIs of miR-34a and miR-335 were frequently methylated in GIST-T1 cells and primary GIST specimens. Transfection of miR-34a or miR-335 mimic molecules into GIST-T1 cells suppressed cell proliferation, and miR-34a also inhibited migration and invasion by GIST-T1 cells. Moreover, miR-34a downregulated a number of predicted target genes, including PDGFRA. RNA interference-mediated knockdown of PDGFRA in GIST-T1 cells suppressed cell proliferation, suggesting the tumor suppressive effect of miR-34a is mediated, at least in part, through targeting PDGFRA.
Conclusions: Our results suggest that miR-34a and miR-335 are candidate tumor suppressive miRNAs in GISTs, and that they are frequent targets of epigenetic silencing in GISTs.
Conflict of interest statement
Figures




References
-
- Wozniak A, Sciot R, Guillou L, Pauwels P, Wasag B, Stul M, et al. Array CGH analysis in primary gastrointestinal stromal tumors: cytogenetic profile correlates with anatomic site and tumor aggressiveness, irrespective of mutational status. Genes Chromosomes Cancer. 2007;46(3):261–76. Epub 2006/12/16. 10.1002/gcc.20408 . - DOI - PubMed
-
- Assamaki R, Sarlomo-Rikala M, Lopez-Guerrero JA, Lasota J, Andersson LC, Llombart-Bosch A, et al. Array comparative genomic hybridization analysis of chromosomal imbalances and their target genes in gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2007;46(6):564–76. Epub 2007/03/03. 10.1002/gcc.20439 . - DOI - PubMed
-
- Igarashi S, Suzuki H, Niinuma T, Shimizu H, Nojima M, Iwaki H, et al. A novel correlation between LINE-1 hypomethylation and the malignancy of gastrointestinal stromal tumors. Clin Cancer Res. 2010;16(21):5114–23. Epub 2010/10/28. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

