Virulence Factors of Streptococcus pneumoniae. Comparison between African and French Invasive Isolates and Implication for Future Vaccines
- PMID: 26214695
- PMCID: PMC4516325
- DOI: 10.1371/journal.pone.0133885
Virulence Factors of Streptococcus pneumoniae. Comparison between African and French Invasive Isolates and Implication for Future Vaccines
Abstract
Background: Many surface proteins thought to promote Streptocococcus pneumoniae virulence have recently been discovered and are currently being considered as future vaccine targets. We assessed the prevalence of 16 virulence genes among 435 S. pneumoniae invasive isolates from France and the "African meningitis belt" region, with particular focus on serotype 1 (Sp1), to compare their geographical distribution, assess their association with site of infection and evaluate their potential interest as new vaccine candidates.
Methods: Detection by PCR of pspA (+families), pspC (+pspC.4), pavA, lytA, phtA,B,D,E, nanA,B,C, rrgA (Pilus-1), sipA (Pilus-2), pcpA and psrp was performed on all isolates, as well as antibiotic resistance testing and MLVA typing (+MLST on 54 representative strains). Determination of ply alleles was performed by sequencing (Sp1 isolates).
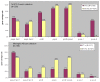
Results: MLVA and virulence genes profiles segregated Sp1 isolates into 2 groups that followed continent distribution. The ply allele 5 and most of the genes that were variable (nanC, Pilus-2, psrp, pcpA, phtD) were present in the French Sp1 isolates (PMEN clone Sweden(1)-28, ST306) but absent from the African ones. Whereas all African Sp1 isolates clustered into a single MLST CC (CC217), MLVA distinguished two CCs that followed temporal evolution. Pilus-2 and psrp were more prevalent in bacteraemic pneumonia yielded isolates and phtB in meningitis-related isolates. Considering vaccine candidates, phtD was less prevalent than anticipated (50%) and pcpA varied importantly between France and Africa (98% versus 34%). Pilus-1 was carried by 7-11% of isolates and associated with β-lactams resistance.
Conclusions: Most virulence genes were carried by the European ST306 clone but were lacking on Sp1 isolates circulating in the African meningitis belt, where a more serious pattern of infection is observed. While virulence proteins are now considered as vaccine targets, the geographical differences in their prevalence could affect the efficacy expected from future vaccines.
Conflict of interest statement
Figures


References
-
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N et al. Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374: 893–902. 10.1016/S0140-6736(09)61204-6 - DOI - PubMed
-
- Black S, Shinefield H, Baxter R, Austrian R, Bracken L, Hansen J et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis 2004; 23:485–9. - PubMed
-
- Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369:1179–86. - PubMed
-
- Simel B, Auranen K, Kaythy H, Goldblatt D, Dagan R, O’Brien K. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 2012; 11:841–855. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

