Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a pilot randomized placebo-controlled trial
- PMID: 26214725
- PMCID: PMC4560666
- DOI: 10.4088/JCP.14m09123
Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a pilot randomized placebo-controlled trial
Abstract
Objective: Obsessive-compulsive disorder (OCD) affects approximately 2.5% of the population and is associated with significant morbidity. Many patients receive little benefit from the best available treatments, and even those who do respond often suffer from significant residual symptoms. Convergent evidence suggests that abnormalities in glutamate homeostasis and neurotransmission may contribute to OCD and that glutamate-modulating medications may be of benefit in patients whose symptoms are refractory to standard interventions. Small open-label trials of augmentation of serotonin reuptake inhibitor (SRI) pharmacotherapy with the glutamate modulator riluzole have suggested benefit in adults with refractory symptoms. We report a pilot randomized placebo-controlled trial of riluzole augmentation of ongoing SRI treatment in SRI-refractory patients.
Method: Outpatients (n = 27) and inpatients (n = 11) with DSM-IV OCD on stable SRI pharmacotherapy were randomized between November 2006 and December 2012 to receive riluzole 50 mg or placebo twice a day and followed for 12 weeks after a 2-week placebo lead-in phase.
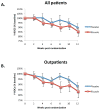
Results: Riluzole was well tolerated; 1 patient experienced moderate nausea, but none discontinued treatment due to side effects. While there was nominally greater Y-BOCS improvement in the riluzole group (our primary outcome) compared to placebo, it did not reach statistical significance. In the outpatient subsample, a trend suggesting benefit from riluzole augmentation for obsessions (P = .056, 2-tailed, uncorrected) was found in a secondary analysis. Among outpatients, more achieved at least a partial response (> 25% improvement) with riluzole than with placebo (P = .02 in a secondary analysis).
Conclusions: Riluzole may be of benefit to a subset of patients. Larger samples would be required to detect effects of the order suggested by the nominal improvement in our outpatient subsample.
Trial registration: ClinicalTrials.gov identifier: NCT00523718.
© Copyright 2015 Physicians Postgraduate Press, Inc.
Figures
References
-
- American Psychiatric Association. and American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5. Washington, D.C: American Psychiatric Association; 2013. p. xliv.p. 947.
-
- Foa EB, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162(1):151–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

