Pragmatic Randomized Trials Without Standard Informed Consent?: A National Survey
- PMID: 26215125
- PMCID: PMC5573142
- DOI: 10.7326/M15-0817
Pragmatic Randomized Trials Without Standard Informed Consent?: A National Survey
Abstract
Background: Significant debate surrounds the issue of whether written consent is necessary for pragmatic randomized, controlled trials (RCTs) with low risk.
Objective: To assess the U.S. public's views on alternatives to written consent for low-risk pragmatic RCTs.
Design: National experimental survey (2 × 2 factorial design) examining support for written consent versus general notification or verbal consent in 2 research scenarios.
Setting: Web-based survey conducted in December 2014.
Participants: 2130 U.S. adults sampled from a nationally representative, probability-based online panel (response rate, 64.0%).
Measurements: Respondent's recommendation to an ethics review board and personal preference as a potential participant on how to obtain consent or notification in the 2 research scenarios.
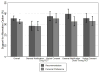
Results: Most respondents in each of the 4 groups (range, 60.3% to 71.5%) recommended written informed consent, and personal preferences were generally in accord with that advice. Most (78.9%) believed that the pragmatic RCTs did not pose additional risks, but 62.5% of these respondents would still recommend written consent. In contrast, a substantial minority in all groups (28.5% to 39.7%) recommended the alternative option (general notification or verbal consent) over written consent.
Limitation: Framing effects could have affected respondents' attitudes, and nonrespondents may have differed in levels of trust toward research or health care institutions.
Conclusion: Most of the public favored written informed consent over the most widely advocated alternatives for low-risk pragmatic RCTs; however, a substantial minority favored general notification or verbal consent.
Primary funding source: Time-sharing Experiments for the Social Sciences and Intramural Research Program of the National Institutes of Health Clinical Center.
Conflict of interest statement
Figures



References
-
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, editors. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press (US); 2013. - PubMed
-
- Sugarman J, Califf RM. Ethics and regulatory complexities for pragmatic clinical trials. JAMA. 2014;311(23):2381–2382. - PubMed
-
- Truog RD, Robinson W, Randolph A, Morris A. Is informed consent always necessary for randomized, controlled trials? N Engl J Med. 1999;340(10):804–807. - PubMed
-
- Faden RR, Kass NE, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. An ethics framework for a learning health care system: a departure from traditional research ethics and clinical ethics. Hastings Cent Rep. 2013 Jan-Feb;41(S1):S16–27. - PubMed
-
- Faden R, Kass N, Whicher D, Stewart W, Tunis S. Ethics and informed consent for comparative effectiveness research with prospective electronic clinical data. Med Care. 2013;51(8 Suppl 3):S53–57. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
