Aversive learning shapes neuronal orientation tuning in human visual cortex
- PMID: 26215466
- PMCID: PMC4518478
- DOI: 10.1038/ncomms8823
Aversive learning shapes neuronal orientation tuning in human visual cortex
Abstract
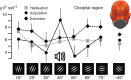
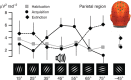
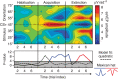
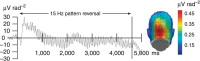
The responses of sensory cortical neurons are shaped by experience. As a result perceptual biases evolve, selectively facilitating the detection and identification of sensory events that are relevant for adaptive behaviour. Here we examine the involvement of human visual cortex in the formation of learned perceptual biases. We use classical aversive conditioning to associate one out of a series of oriented gratings with a noxious sound stimulus. After as few as two grating-sound pairings, visual cortical responses to the sound-paired grating show selective amplification. Furthermore, as learning progresses, responses to the orientations with greatest similarity to the sound-paired grating are increasingly suppressed, suggesting inhibitory interactions between orientation-selective neuronal populations. Changes in cortical connectivity between occipital and fronto-temporal regions mirror the changes in visuo-cortical response amplitudes. These findings suggest that short-term behaviourally driven retuning of human visual cortical neurons involves distal top-down projections as well as local inhibitory interactions.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

