Loss of DJ-1 elicits retinal abnormalities, visual dysfunction, and increased oxidative stress in mice
- PMID: 26215528
- PMCID: PMC4573318
- DOI: 10.1016/j.exer.2015.07.014
Loss of DJ-1 elicits retinal abnormalities, visual dysfunction, and increased oxidative stress in mice
Abstract
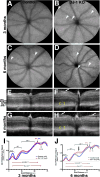
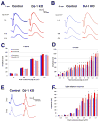
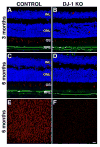
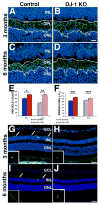
DJ-1/PARK7 mutations or deletions cause autosomal recessive early onset Parkinson's disease (PD). Thus, DJ-1 protein has been extensively studied in brain and neurons. PD patients display visual symptoms; however, the visual symptoms specifically attributed to PD patients carrying DJ-1/PARK7 mutations are not known. In this study, we analyzed the structure and physiology of retinas of 3- and 6-month-old DJ-1 knockout (KO) mice to determine how loss of function of DJ-1 specifically contributes to the phenotypes observed in PD patients. As compared to controls, the DJ-1 KO mice displayed an increase in the amplitude of the scotopic ERG b-wave and cone ERG, while the amplitude of a subset of the dc-ERG components was decreased. The main structural changes in the DJ-1 KO retinas were found in the outer plexiform layer (OPL), photoreceptors and retinal pigment epithelium (RPE), which were observed at 3 months and progressively increased at 6 months. RPE thinning and structural changes within the OPL were observed in the retinas in DJ-1 KO mice. DJ-1 KO retinas also exhibited disorganized outer segments, central decrease in red/green cone opsin staining, decreased labeling of ezrin, broader distribution of ribeye labeling, decreased tyrosine hydroxylase in dopaminergic neurons, and increased 7,8-dihydro-8-oxoguanine-labeled DNA oxidation. Accelerated outer retinal atrophy was observed in DJ-1 KO mice after selective oxidative damage induced by a single tail vein injection of NaIO3, exposing increased susceptibility to oxidative stress. Our data indicate that DJ-1-deficient retinas exhibit signs of morphological abnormalities and physiological dysfunction in association with increased oxidative stress. Degeneration of RPE cells in association with oxidative stress is a key hallmark of age-related macular degeneration (AMD). Therefore, in addition to detailing the visual defects that occur as a result of the absence of DJ-1, our data is also relevant to AMD pathogenesis.
Keywords: Biochemistry; DJ-1 knockout; Histology; Immunohistology; Morphology; Oxidation; Physiology; Retina.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures





References
-
- Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol. 2003;54:283–286. - PubMed
-
- Awatramani G, Wang J, Slaughter MM. Amacrine and ganglion cell contributions to the electroretinogram in amphibian retina. Vis Neurosci. 2001;18:147–156. - PubMed
-
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. - PubMed
-
- Bell BA, Kaul C, Rayborn ME, Hollyfield JG. Baseline imaging reveals preexisting retinal abnormalities in mice. Adv Exp Med Biol. 2012;723:459–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

