miR-431 promotes differentiation and regeneration of old skeletal muscle by targeting Smad4
- PMID: 26215566
- PMCID: PMC4536309
- DOI: 10.1101/gad.263574.115
miR-431 promotes differentiation and regeneration of old skeletal muscle by targeting Smad4
Abstract
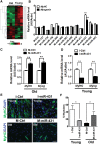
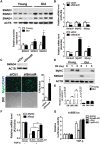
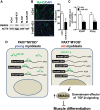
The myogenic capacity of myoblasts decreases in skeletal muscle with age. In addition to environmental factors, intrinsic factors are important for maintaining the regenerative potential of muscle progenitor cells, but their identities are largely unknown. Here, comparative analysis of microRNA (miRNA) expression profiles in young and old myoblasts uncovered miR-431 as a novel miRNA showing markedly reduced abundance in aged myoblasts. Importantly, elevating miR-431 improved the myogenic capacity of old myoblasts, while inhibiting endogenous miR-431 lowered myogenesis. Bioinformatic and biochemical analyses revealed that miR-431 directly interacted with the 3' untranslated region (UTR) of Smad4 mRNA, which encodes one of the downstream effectors of TGF-β signaling. In keeping with the low levels of miR-431 in old myoblasts, SMAD4 levels increased in this myoblast population. Interestingly, in an in vivo model of muscle regeneration following cardiotoxin injury, ectopic miR-431 injection greatly improved muscle regeneration and reduced SMAD4 levels. Consistent with the finding that the mouse miR-431 seed sequence in the Smad4 3' UTR is conserved in the human SMAD4 3' UTR, inhibition of miR-431 also repressed the myogenic capacity of human skeletal myoblasts. Taken together, our results suggest that the age-associated miR-431 plays a key role in maintaining the myogenic ability of skeletal muscle with age.
Keywords: SMAD4; differentiation; miR-431; muscle aging; myoblast; regeneration.
© 2015 Lee et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



Similar articles
-
microRNA for determining the age-related myogenic capabilities of skeletal muscle.BMB Rep. 2015 Nov;48(11):595-6. doi: 10.5483/bmbrep.2015.48.11.211. BMB Rep. 2015. PMID: 26521942 Free PMC article.
-
miR-26a is required for skeletal muscle differentiation and regeneration in mice.Genes Dev. 2012 Oct 1;26(19):2180-91. doi: 10.1101/gad.198085.112. Genes Dev. 2012. PMID: 23028144 Free PMC article.
-
MicroRNA-17-92 regulates myoblast proliferation and differentiation by targeting the ENH1/Id1 signaling axis.Cell Death Differ. 2016 Oct;23(10):1658-69. doi: 10.1038/cdd.2016.56. Epub 2016 Jun 17. Cell Death Differ. 2016. PMID: 27315298 Free PMC article.
-
MicroRNAs involved in skeletal muscle differentiation.J Genet Genomics. 2013 Mar 20;40(3):107-16. doi: 10.1016/j.jgg.2013.02.002. Epub 2013 Feb 20. J Genet Genomics. 2013. PMID: 23522383 Review.
-
Effects of microRNAs on skeletal muscle development.Gene. 2018 Aug 20;668:107-113. doi: 10.1016/j.gene.2018.05.039. Epub 2018 May 25. Gene. 2018. PMID: 29775754 Review.
Cited by
-
MicroRNAs in Skeletal Muscle Aging: Current Issues and Perspectives.J Gerontol A Biol Sci Med Sci. 2019 Jun 18;74(7):1008-1014. doi: 10.1093/gerona/gly207. J Gerontol A Biol Sci Med Sci. 2019. PMID: 30215687 Free PMC article. Review.
-
NADPH Oxidase 4 Contributes to Myoblast Fusion and Skeletal Muscle Regeneration.Oxid Med Cell Longev. 2019 Nov 18;2019:3585390. doi: 10.1155/2019/3585390. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31827673 Free PMC article.
-
miR-208b modulating skeletal muscle development and energy homoeostasis through targeting distinct targets.RNA Biol. 2020 May;17(5):743-754. doi: 10.1080/15476286.2020.1728102. Epub 2020 Feb 24. RNA Biol. 2020. PMID: 32037961 Free PMC article.
-
miR-9-5p promotes myogenic differentiation via the Dlx3/Myf5 axis.PeerJ. 2022 May 3;10:e13360. doi: 10.7717/peerj.13360. eCollection 2022. PeerJ. 2022. PMID: 35529491 Free PMC article.
-
Molecular mechanisms and therapeutic interventions in sarcopenia.Osteoporos Sarcopenia. 2017 Sep;3(3):117-122. doi: 10.1016/j.afos.2017.08.098. Epub 2017 Sep 27. Osteoporos Sarcopenia. 2017. PMID: 30775515 Free PMC article. Review.
References
-
- Ambros V. 2004. The functions of animal microRNAs. Nature 431: 350–355. - PubMed
-
- Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. - PubMed
-
- Brack AS, Bildsoe H, Hughes SM. 2005. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci 118: 4813–4821. - PubMed
-
- Callis TE, Chen JF, Wang DZ. 2007. MicroRNAs in skeletal and cardiac muscle development. DNA Cell Biol 26: 219–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
