Paracrine signaling between tumor subclones of mouse SCLC: a critical role of ETS transcription factor Pea3 in facilitating metastasis
- PMID: 26215568
- PMCID: PMC4536306
- DOI: 10.1101/gad.262998.115
Paracrine signaling between tumor subclones of mouse SCLC: a critical role of ETS transcription factor Pea3 in facilitating metastasis
Abstract
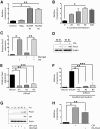
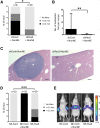
Tumor heterogeneity can create a unique symbiotic tumor microenvironment. Earlier, we showed that clonal evolution in mouse small cell lung cancer (SCLC) can result in subclones that, upon cografting, endow the neuroendocrine tumor cells with metastatic potential. We now show that paracrine signaling between SCLC subclones is a critical requirement in the early steps of the metastatic process, such as local invasion and intravasation. We further show evidence that paracrine signaling via fibroblast growth factor 2 (Fgf2) and Mapk between these diverged tumor subclones causes enhanced expression of the Pea3 (polyomavirus enhancer activator 3) transcription factor, resulting in metastatic dissemination of the neuroendocrine tumor subclones. Our data reveal for the first time paracrine signaling between tumor cell subclones in SCLC that results in metastatic spread of SCLC.
Keywords: Fgf2; Pea3; metastasis; small cell lung cancer; tumor heterogeneity.
© 2015 Kwon et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


Comment in
-
Tumor heterogeneity-induced signaling regulates SCLC metastasis.Cell Cycle. 2015;14(21):3347-8. doi: 10.1080/15384101.2015.1093445. Cell Cycle. 2015. PMID: 26506985 Free PMC article. No abstract available.
References
-
- Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM, Kempski H, Moorman AV, Titley I, Swansbury J, et al. 2011. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 469: 356–361. - PubMed
-
- Aytes A, Mitrofanova A, Kinkade CW, Lefebvre C, Lei M, Phelan V, LeKaye HC, Koutcher JA, Cardiff RD, Califano A, et al. 2013. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proc Natl Acad Sci 110: E3506–E3515. - PMC - PubMed
-
- Brent AE, Tabin CJ. 2004. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131: 3885–3896. - PubMed
-
- Calbo J, van Montfort E, Proost N, van Drunen E, Beverloo HB, Meuwissen R, Berns A. 2011. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 19: 244–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
