RHOBTB3 promotes proteasomal degradation of HIFα through facilitating hydroxylation and suppresses the Warburg effect
- PMID: 26215701
- PMCID: PMC4559813
- DOI: 10.1038/cr.2015.90
RHOBTB3 promotes proteasomal degradation of HIFα through facilitating hydroxylation and suppresses the Warburg effect
Abstract
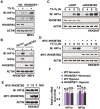
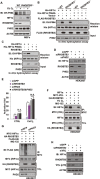
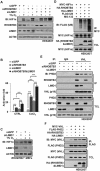
Hypoxia-inducible factors (HIFs) are master regulators of adaptive responses to low oxygen, and their α-subunits are rapidly degraded through the ubiquitination-dependent proteasomal pathway after hydroxylation. Aberrant accumulation or activation of HIFs is closely linked to many types of cancer. However, how hydroxylation of HIFα and its delivery to the ubiquitination machinery are regulated remains unclear. Here we show that Rho-related BTB domain-containing protein 3 (RHOBTB3) directly interacts with the hydroxylase PHD2 to promote HIFα hydroxylation. RHOBTB3 also directly interacts with the von Hippel-Lindau (VHL) protein, a component of the E3 ubiquitin ligase complex, facilitating ubiquitination of HIFα. Remarkably, RHOBTB3 dimerizes with LIMD1, and constructs a RHOBTB3/LIMD1-PHD2-VHL-HIFα complex to effect the maximal degradation of HIFα. Hypoxia reduces the RHOBTB3-centered complex formation, resulting in an accumulation of HIFα. Importantly, the expression level of RHOBTB3 is greatly reduced in human renal carcinomas, and RHOBTB3 deficiency significantly elevates the Warburg effect and accelerates xenograft growth. Our work thus reveals that RHOBTB3 serves as a scaffold to organize a multi-subunit complex that promotes the hydroxylation, ubiquitination and degradation of HIFα.
Figures




References
-
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. - PubMed
-
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. - PubMed
-
- Semenza GL, Jiang BH, Leung SW, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

