Shape descriptors of the "never resting" microglia in three different acute brain injury models in mice
- PMID: 26215806
- PMCID: PMC4513020
- DOI: 10.1186/s40635-015-0039-0
Shape descriptors of the "never resting" microglia in three different acute brain injury models in mice
Abstract
Background: The study of microglia and macrophage (M/M) morphology represents a key tool to understand the functional activation state and the pattern of distribution of these cells in acute brain injury. The identification of reliable quantitative morphological parameters is urgently needed to understand these cell roles in brain injury and to explore strategies aimed at therapeutically manipulating the inflammatory response.
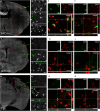
Methods: We used three different clinically relevant murine models of focal injury, namely, controlled cortical impact brain injury (traumatic brain injury (TBI)) and transient and permanent occlusion of middle cerebral artery (tMCAo and pMCAo, respectively). Twenty-four hours after injury, M/M cells were labeled by CD11b, and ×40 photomicrographs were acquired by unbiased sampling of the lesion core using a motorized stage microscope. Images were processed with Fiji software to obtain shape descriptors.
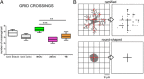
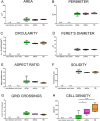
Results: We validated several parameters, including area, perimeter, Feret's diameter (caliper), circularity, aspect ratio, and solidity, providing quantitative information on M/M morphology over wide tissue portions. We showed that the shape descriptors that best represent M/M ramification/elongation are area and perimeter, while circularity and solidity provide information on the ameboid shape. We also provide evidence of the involvement of different populations in local inflammatory events, with macrophages replacing microglia into the lesion core when reperfusion does not occur. Analysis of CD45(high)+ cell morphology, whose shape does not change, did not yield any difference, thus confirming the reliability of the approach.
Conclusions: We have defined specific morphological features that M/M acquire in response to different acute insults by applying a sensitive and readily applicable approach to cell morphological analysis in the brain tissue. Potential application of this method can be extended to all cell types able to change shape following activation, e.g., astrocytes, or to different disease states, including chronic pathologies.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

