Critical illness-induced bone loss is related to deficient autophagy and histone hypomethylation
- PMID: 26215816
- PMCID: PMC4480347
- DOI: 10.1186/s40635-015-0052-3
Critical illness-induced bone loss is related to deficient autophagy and histone hypomethylation
Abstract
Background: Survivors of critical illness are at increased risk of fractures. This may be due to increased osteoclast formation during critical illness, leading to trabecular bone loss. Such bone loss has also been observed in Paget's disease, and has been related to deficient autophagy. Deficient autophagy has also been documented in vital organs and skeletal muscle of critically ill patients. The objective of this study was to investigate whether deficient autophagy can be linked to critical illness-induced bone loss.
Methods: Osteoclasts grown in vitro and their precursor cells isolated from peripheral blood of critically ill patients and from matched healthy volunteers were analysed for the expression of autophagy genes (SQSTM1, Atg3 and Atg7), and proteins (p62, Atg-5, and microtubule-associated protein light chain 3-II (LC3-II)) and for autophagy and epigenetic signalling factors via PCR arrays and were treated with the autophagy inducer rapamycin. The effect of rapamycin was also investigated at the tissue level in an in vivo rabbit model of critical illness.
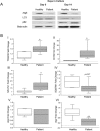
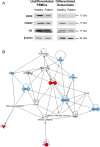
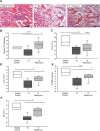
Results: Many more osteoclasts formed in vitro from the blood precursor cells isolated from critically ill patients, which accumulated p62, and displayed reduced expression of Atg5, Atg7, and LC3-II compared to healthy controls, suggesting deficient autophagy, whilst addition of rapamycin reduced osteoclast formation. PCR arrays revealed a down-regulation of histone methyltransferases coupled with an up-regulation of negative regulators of autophagy. Critically ill rabbits displayed a reduction in trabecular and cortical bone, which was rescued with rapamycin.
Conclusions: Deficient autophagy in osteoclasts and their blood precursor cells at least partially explained aberrant osteoclast formation during critical illness and was linked to global histone hypomethylation. Treatment with the autophagy activator Rapamycin reduced patient osteoclast formation in vitro and reduced the amount of bone loss in critically ill rabbits in vivo. These findings may help to develop novel therapeutic targets to prevent critical illness-induced bone loss.
Figures


References
-
- Vanhees I, Gunst J, Janssens T, Wauters A, Van Herck E, Van Cromphaut S, Van den Berghe G, Owen HC. Enhanced immunoreceptor tyrosine-based activation motif signaling is related to pathological bone resorption during critical illness. Horm Metab Res. 2013;45:862–9. doi: 10.1055/s-0033-1351290. - DOI - PubMed
-
- Hocking LJ, Lucas GJ, Daroszewska A, Mangion J, Olavesen M, Cundy T, Nicholson GC, Ward L, Bennett ST, Wuyts W, Van Hul W, Ralston SH. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735–9. doi: 10.1093/hmg/11.22.2735. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

