Water-soluble lipophilic MR contrast agents for cell membrane labeling
- PMID: 26215869
- PMCID: PMC4546878
- DOI: 10.1007/s00775-015-1280-4
Water-soluble lipophilic MR contrast agents for cell membrane labeling
Abstract
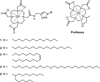
Long-term cell tracking using MR imaging necessitates the development of contrast agents that both label and are retained by cells. One promising strategy for long-term cell labeling is the development of lipophilic Gd(III)-based contrast agents that anchor into the cell membrane. We have previously reported the efficacy of monomeric and multimeric lipophilic agents and showed that the monomeric agents have improved labeling and contrast enhancement of cell populations. Here, we report on the synthesis, characterization, and in vitro testing of a series of monomeric lipophilic contrast agents with varied alkyl chain compositions. We show that these agents disperse in water, localize to the cell membrane, and label HeLa and MCF7 cells effectively. Additionally, these agents have up to tenfold improved retention in cells compared to clinically available ProHance(®).
Figures
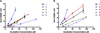


References
-
- Valone FH, Small E, MacKenzie M, Burch P, Lacy M, Peshwa MV, Laus R. Cancer J. 2001;7:S53–S61. - PubMed
-
- Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L, Gomez-Bueno M, Cantalapiedra A, Fernandez J, Gutierrez O, Sanchez PL, Hernandez C, Sanz R, Garcia-Sancho J, Sanchez A. Circulation research. 2004;95:742–748. - PubMed
-
- Semsarian C. Internal medicine journal. 2002;32:259–265. - PubMed
-
- Petit-Zeman S. Nature biotechnology. 2001;19:201–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

