Manganese-Enhanced Magnetic Resonance Imaging Enables In Vivo Confirmation of Peri-Infarct Restoration Following Stem Cell Therapy in a Porcine Ischemia-Reperfusion Model
- PMID: 26215972
- PMCID: PMC4608088
- DOI: 10.1161/JAHA.115.002044
Manganese-Enhanced Magnetic Resonance Imaging Enables In Vivo Confirmation of Peri-Infarct Restoration Following Stem Cell Therapy in a Porcine Ischemia-Reperfusion Model
Abstract
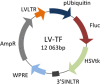
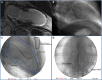
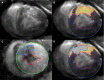
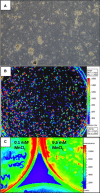
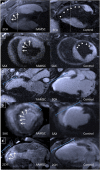
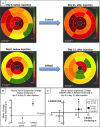
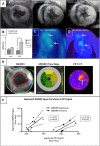
Background: The exact mechanism of stem cell therapy in augmenting the function of ischemic cardiomyopathy is unclear. In this study, we hypothesized that increased viability of the peri-infarct region (PIR) produces restorative benefits after stem cell engraftment. A novel multimodality imaging approach simultaneously assessed myocardial viability (manganese-enhanced magnetic resonance imaging [MEMRI]), myocardial scar (delayed gadolinium enhancement MRI), and transplanted stem cell engraftment (positron emission tomography reporter gene) in the injured porcine hearts.
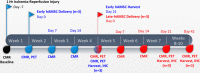
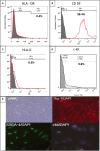
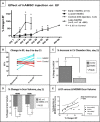
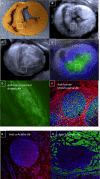
Methods and results: Twelve adult swine underwent ischemia-reperfusion injury. Digital subtraction of MEMRI-negative myocardium (intrainfarct region) from delayed gadolinium enhancement MRI-positive myocardium (PIR and intrainfarct region) clearly delineated the PIR in which the MEMRI-positive signal reflected PIR viability. Human amniotic mesenchymal stem cells (hAMSCs) represent a unique population of immunomodulatory mesodermal stem cells that restored the murine PIR. Immediately following hAMSC delivery, MEMRI demonstrated an increased PIR viability signal compared with control. Direct PIR viability remained higher in hAMSC-treated hearts for >6 weeks. Increased PIR viability correlated with improved regional contractility, left ventricular ejection fraction, infarct size, and hAMSC engraftment, as confirmed by immunocytochemistry. Increased MEMRI and positron emission tomography reporter gene signal in the intrainfarct region and the PIR correlated with sustained functional augmentation (global and regional) within the hAMSC group (mean change, left ventricular ejection fraction: hAMSC 85±60%, control 8±10%; P<0.05) and reduced chamber dilatation (left ventricular end-diastole volume increase: hAMSC 24±8%, control 110±30%; P<0.05).
Conclusions: The positron emission tomography reporter gene signal of hAMSC engraftment correlates with the improved MEMRI signal in the PIR. The increased MEMRI signal represents PIR viability and the restorative potential of the injured heart. This in vivo multimodality imaging platform represents a novel, real-time method of tracking PIR viability and stem cell engraftment while providing a mechanistic explanation of the therapeutic efficacy of cardiovascular stem cells.
Keywords: ischemia–reperfusion injury; magnetic resonance imaging; manganese‐enhanced magnetic resonance imaging; peri‐infarct region imaging; stem cell imaging.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures
References
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. - PubMed
-
- Heidary S, Patel H, Chung J, Yokota H, Gupta SN, Bennett MV, Katikireddy C, Nguyen P, Pauly JM, Terashima M, McConnell MV, Yang PC. Quantitative tissue characterization of infarct core and border zone in patients with ischemic cardiomyopathy by magnetic resonance is associated with future cardiovascular events. J Am Coll Cardiol. 2010;55:2762–2768. - PubMed
-
- Tsukiji M, Nguyen P, Narayan G, Hellinger J, Chan F, Herfkens R, Pauly JM, McConnell MV, Yang PC. Peri-infarct ischemia determined by cardiovascular magnetic resonance evaluation of myocardial viability and stress perfusion predicts future cardiovascular events in patients with severe ischemic cardiomyopathy. J Cardiovasc Magn Reson. 2006;8:773–779. - PubMed
-
- Jaussaud J, Biais M, Calderon J, Chevaleyre J, Duchez P, Ivanovic Z, Couffinhal T, Barandon L. Hypoxia-preconditioned mesenchymal stromal cells improve cardiac function in a swine model of chronic myocardial ischaemia. Eur J Cardiothorac Surg. 2012;43:1050–1057. - PubMed
-
- Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–S37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

