ANISERP: a new serpin from the parasite Anisakis simplex
- PMID: 26215984
- PMCID: PMC4517634
- DOI: 10.1186/s13071-015-1006-z
ANISERP: a new serpin from the parasite Anisakis simplex
Abstract
Background: Serine proteinase inhibitors (serpins) finely regulate serine proteinase activity via a suicide substrate-like inhibitory mechanism. In parasitic nematodes, some serpins interact with host physiological processes; however, little is known about these essential molecules in Anisakis. This article reports the gene sequencing, cloning, expression and preliminary biochemical and bioinformatically-based structural characterization of a new Anisakis serpin (ANISERP).
Methods: The full AniSerp gene was cloned by specific RACE-PCR after screening an Anisakis simplex (L3) cDNA library. For biochemical assays, the AniSerp gene was subcloned into both prokaryotic and eukaryotic vectors, and the recombinant proteins were purified. The inhibitory properties of the proteins were tested in classical biochemical assays using human serine peptidases and AMC substrates. Immunolocalization of ANISERP, theoretical structural analysis and bioinformatically-based structural modelling of the ANISERP protein were also conducted.
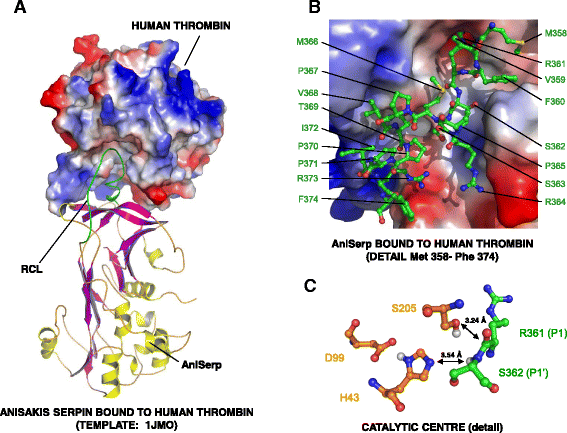
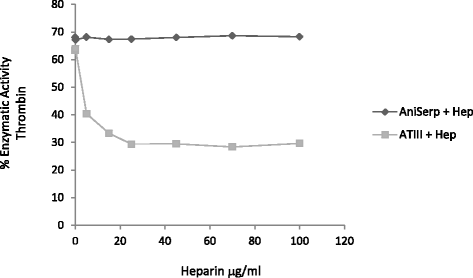
Results: The AniSerp gene was found to have 1194 nucleotides, coding for a protein of 397 amino acid residues plus a putative N-terminal signal peptide. It showed significant similarity to other nematode, arthropod and mammalian serpins. The recombinant ANISERP expressed in the prokaryotic and eukaryotic systems inhibited the human serine proteases thrombin, trypsin and cathepsin G in a concentration-dependent manner. No inhibitory activity against Factor Xa, Factor XIa, Factor XIIa, elastase, plasmin or chymotrypsin was observed. ANISERP also acted on the cysteine protease cathepsin L. ANISERP was mainly localized in the nematode pseudocoelomic fluid, somatic muscle cell bodies and intestinal cells. The findings of molecular dynamics studies suggest that ANISERP inhibits thrombin via a suicide substrate-like inhibitory mechanism, similar to the mechanism of action of mammalian coagulation inhibitors. In contrast to findings concerning human antithrombin III, heparin had no effect on ANISERP anticoagulant inhibitory activity.
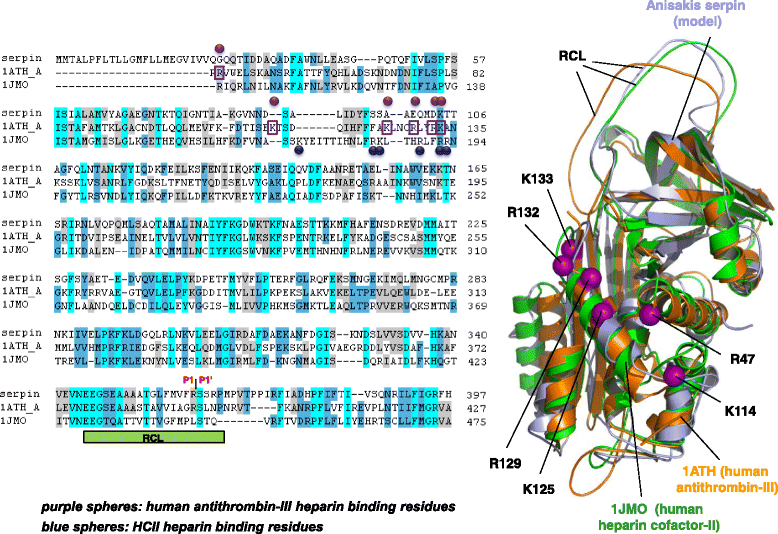
Conclusions: Our findings suggest that ANISERP is an internal Anisakis regulatory serpin and that the inhibitory activity against thrombin depends on a suicide substrate-like inhibitory mechanism, similar to that described for human antithrombin (AT)-III. The fact that heparin does not modulate the anticoagulant activity of ANISERP might be explained by the absence in the latter of five of the six positively charged residues usually seen at the AT-III-heparin binding site.
Figures


References
-
- Audícana MT, del Pozo MD, Iglesias R, Ubeira FM. Anisakis simplex and Pseudoterranova decipiens. In: Miliotis M, Bier J, editors. International handbook on foodborne pathogens. New York: Marcell Dekker; 2003. pp. 613–36.
-
- Bier JW. Anisakiasis. Laboratory diagnosis of infectious diseases. In: Balows A, Hausler WJ Jr, Ohashi M, Turano A, editors. Bacterial, mycotic and parasitic diseases. New York: Springer; 1988. pp. 768–74.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

