Monoglyceride lipase: Structure and inhibitors
- PMID: 26216043
- PMCID: PMC4728057
- DOI: 10.1016/j.chemphyslip.2015.07.011
Monoglyceride lipase: Structure and inhibitors
Abstract
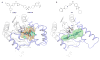
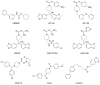
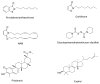
Monoglyceride lipase (MGL), the main enzyme responsible for the hydrolytic deactivation of the endocannabinoid 2-arachidonoyl-sn-glycerol (2-AG), is an intracellular serine hydrolase that plays critical roles in many physiological and pathological processes, such as pain, inflammation, neuroprotection and cancer. The crystal structures of MGL that are currently available provide valuable information about how this enzyme might function and interact with site-directed small-molecule inhibitors. On the other hand, its conformational equilibria and the contribution of regulatory cysteine residues present within the substrate-binding pocket or on protein surface remain open issues. Several classes of MGL inhibitors have been developed, from early reversible ones, such as URB602 and pristimerin, to carbamoylating agents that react with the catalytic serine, such as JZL184 and more recent O-hexafluoroisopropyl carbamates. Other inhibitors that modulate MGL activity by interacting with conserved regulatory cysteines act through mechanisms that deserve to be more thoroughly investigated.
Keywords: 2-Arachidonoyl-sn-glycerol (2-AG); Cysteines; Endocannabinoids; Inhibitors; Lid domain; Monoglyceride lipase (MGL).
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures
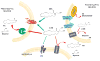

References
-
- Aaltonen N, Savinainen JR, Ribas CR, Rönkkö J, Kuusisto A, Korhonen J, Navia-Paldanius D, Häyrinen J, Takabe P, Käsnänen H, Pantsar T, Laitinen T, Lehtonen M, Pasonen-Seppänen S, Poso A, Nevalainen T, Laitinen JT. Piperazine and piperidine triazole ureas as ultrapotent and highly selective inhibitors of monoacylglycerol lipase. Chem Biol. 2013;20:79–90. - PubMed
-
- Aguado Y, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzmán M, Galve-Roperh I. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. - PubMed
-
- Aloulou A, Rodriguez JA, Fernandez S, van Oosterhout D, Puccinelli D, Carrière F. Exploring the specific features of interfacial enzymology based on lipase studies. Biochim Biophys Acta. 2006;1761:995–1013. - PubMed
-
- Beltramo M, Piomelli D. Carrier-mediated and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonoylglycerol. Neuroreport. 2000;11:1231–1235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

