CD68 acts as a major gateway for malaria sporozoite liver infection
- PMID: 26216124
- PMCID: PMC4548058
- DOI: 10.1084/jem.20110575
CD68 acts as a major gateway for malaria sporozoite liver infection
Abstract
After being delivered by the bite from an infected mosquito, Plasmodium sporozoites enter the blood circulation and infect the liver. Previous evidence suggests that Kupffer cells, a macrophage-like component of the liver blood vessel lining, are traversed by sporozoites to initiate liver invasion. However, the molecular determinants of sporozoite-Kupffer cell interactions are unknown. Understanding the molecular basis for this specific recognition may lead to novel therapeutic strategies to control malaria. Using a phage display library screen, we identified a peptide, P39, that strongly binds to the Kupffer cell surface and, importantly, inhibits sporozoite Kupffer cell entry. Furthermore, we determined that P39 binds to CD68, a putative receptor for sporozoite invasion of Kupffer cells that acts as a gateway for malaria infection of the liver.
© 2015 Cha et al.
Figures
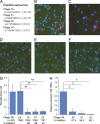
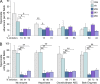

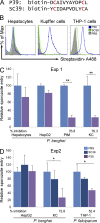





Comment in
-
Gateway to deLiver: How malaria sporozoites cross the sinusoidal barrier.J Exp Med. 2015 Aug 24;212(9):1340. doi: 10.1084/jem.2129insight1. J Exp Med. 2015. PMID: 26304979 Free PMC article. No abstract available.
References
-
- Bruña-Romero O., Hafalla J.C., González-Aseguinolaza G., Sano G., Tsuji M., and Zavala F.. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499–1502. 10.1016/S0020-7519(01)00265-X - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

