A Historical Perspective on the Identification of Cell Types in Pancreatic Islets of Langerhans by Staining and Histochemical Techniques
- PMID: 26216133
- PMCID: PMC4530402
- DOI: 10.1369/0022155415589119
A Historical Perspective on the Identification of Cell Types in Pancreatic Islets of Langerhans by Staining and Histochemical Techniques
Abstract
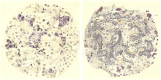
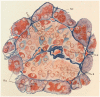
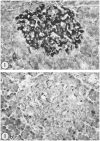
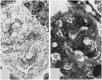
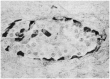
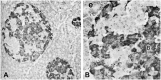
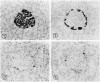
Before the middle of the previous century, cell types of the pancreatic islets of Langerhans were identified primarily on the basis of their color reactions with histological dyes. At that time, the chemical basis for the staining properties of islet cells in relation to the identity, chemistry and structure of their hormones was not fully understood. Nevertheless, the definitive islet cell types that secrete glucagon, insulin, and somatostatin (A, B, and D cells, respectively) could reliably be differentiated from each other with staining protocols that involved variations of one or more tinctorial techniques, such as the Mallory-Heidenhain azan trichrome, chromium hematoxylin and phloxine, aldehyde fuchsin, and silver impregnation methods, which were popularly used until supplanted by immunohistochemical techniques. Before antibody-based staining methods, the most bona fide histochemical techniques for the identification of islet B cells were based on the detection of sulfhydryl and disulfide groups of insulin. The application of the classical islet tinctorial staining methods for pathophysiological studies and physiological experiments was fundamental to our understanding of islet architecture and the physiological roles of A and B cells in glucose regulation and diabetes.
Keywords: beta cells; diabetes; glucagon; immunocytochemistry; immunohistochemistry; insulin; islet cells; pancreas; somatostatin; staining.
© The Author(s) 2015.
Conflict of interest statement
Figures





References
-
- Ahrén B, Wierup N, Sundler F. (2006). Neuropeptides and the regulation of islet function. Diabetes 55: S98-S107.
-
- Andralojc KM, Mercalli A, Nowak KW, Albarello L, Calcagno R, Luzi L, Bonifacio E, Doglioni C, Piemonti L. (2009). Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 52:486-493. - PubMed
-
- Andrew A. (1976). An experimental investigation into the possible neural crest origin of pancreatic APUD (islet) cells. J Embrol Exp Morphol 35:577-593. - PubMed
-
- Bangle R., Jr (1954). Gomori’s paraldehyde-fuchsin stain. I. Physico-chemical and staining properties of the dye. J Histochem Cytochem 2:62-67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

