Characterization of Antibodies to Products of Proinsulin Processing Using Immunofluorescence Staining of Pancreas in Multiple Species
- PMID: 26216140
- PMCID: PMC4530395
- DOI: 10.1369/0022155415576541
Characterization of Antibodies to Products of Proinsulin Processing Using Immunofluorescence Staining of Pancreas in Multiple Species
Abstract
The efficient processing of proinsulin into mature insulin and C-peptide is often compromised under conditions of beta cell stress, including diabetes. Impaired proinsulin processing has been challenging to examine by immunofluorescence staining in pancreas tissue because the characterization of antibodies specific for proinsulin, proinsulin intermediates, processed insulin and C-peptide has been limited. This study aimed to identify and characterize antibodies that can be used to detect products of proinsulin processing by immunofluorescence staining in pancreata from different species (mice, rats, dog, pig and human). We took advantage of several knockout mouse lines that lack either an enzyme involved in proinsulin processing or an insulin gene. Briefly, we report antibodies that are specific for several proinsulin processing products, including: a) insulin or proinsulin that has been appropriately processed at the B-C junction; b) proinsulin with a non-processed B-C junction; c) proinsulin with a non-processed A-C junction; d) rodent-specific C-peptide 1; e) rodent-specific C-peptide 2; and f) human-specific C-peptide or proinsulin. In addition, we also describe two 'pan-insulin' antibodies that react with all forms of insulin and proinsulin intermediates, regardless of the species. These antibodies are valuable tools for studying proinsulin processing by immunofluorescence staining and distinguishing between proinsulin products in different species.
Keywords: beta cells; diabetes; immunofluorescence staining; islets; proinsulin processing.
© The Author(s) 2015.
Conflict of interest statement
Figures




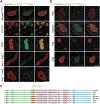
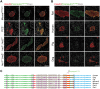




References
-
- Bosco D, Rouiller DG, Halban PA. (2007). Differential expression of E-cadherin at the surface of rat beta-cells as a marker of functional heterogeneity. J Endocrinol 194:21-29. - PubMed
-
- Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF. (1998). Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem 273:3431-3437. - PubMed
-
- Goodge KA, Hutton JC. (2000). Translational regulation of proinsulin biosynthesis and proinsulin conversion in the pancreatic beta-cell. Sem Cell Dev Biol 11:235-242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

