Double strand break repair by capture of retrotransposon sequences and reverse-transcribed spliced mRNA sequences in mouse zygotes
- PMID: 26216318
- PMCID: PMC4516963
- DOI: 10.1038/srep12281
Double strand break repair by capture of retrotransposon sequences and reverse-transcribed spliced mRNA sequences in mouse zygotes
Abstract
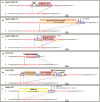
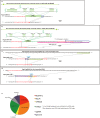
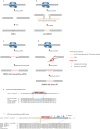
The CRISPR/Cas system efficiently introduces double strand breaks (DSBs) at a genomic locus specified by a single guide RNA (sgRNA). The DSBs are subsequently repaired through non-homologous end joining (NHEJ) or homologous recombination (HR). Here, we demonstrate that DSBs introduced into mouse zygotes by the CRISPR/Cas system are repaired by the capture of DNA sequences deriving from retrotransposons, genomic DNA, mRNA and sgRNA. Among 93 mice analysed, 57 carried mutant alleles and 22 of them had long de novo insertion(s) at DSB-introduced sites; two were spliced mRNAs of Pcnt and Inadl without introns, indicating the involvement of reverse transcription (RT). Fifteen alleles included retrotransposons, mRNAs, and other sequences without evidence of RT. Two others were sgRNAs with one containing T7 promoter-derived sequence suggestive of a PCR product as its origin. In conclusion, RT-product-mediated DSB repair (RMDR) and non-RMDR repair were identified in the mouse zygote. We also confirmed that both RMDR and non-RMDR take place in CRISPR/Cas transfected NIH-3T3 cells. Finally, as two de novo MuERV-L insertions in C57BL/6 mice were shown to have characteristic features of RMDR in natural conditions, we hypothesize that RMDR contributes to the emergence of novel DNA sequences in the course of evolution.
Figures

References
-
- Deininger P. L., Moran J. V., Batzer M. A. & Kazazian H. H. Jr. Mobile elements and mammalian genome evolution. Curr Opin Genet Dev 13, 651–658 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

