Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment
- PMID: 26216631
- PMCID: PMC4662416
- DOI: 10.1016/bs.acr.2015.04.002
Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment
Abstract
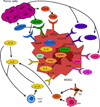
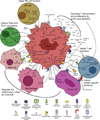
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that suppress innate and adaptive immunity. MDSCs are present in many disease settings; however, in cancer, they are a major obstacle for both natural antitumor immunity and immunotherapy. Tumor and host cells in the tumor microenvironment (TME) produce a myriad of pro-inflammatory mediators that activate MDSCs and drive their accumulation and suppressive activity. MDSCs utilize a variety of mechanisms to suppress T cell activation, induce other immune-suppressive cell populations, regulate inflammation in the TME, and promote the switching of the immune system to one that tolerates and enhances tumor growth. Because MDSCs are present in most cancer patients and are potent immune-suppressive cells, MDSCs have been the focus of intense research in recent years. This review describes the history and identification of MDSCs, the role of inflammation and intracellular signaling events governing MDSC accumulation and suppressive activity, immune-suppressive mechanisms utilized by MDSCs, and recent therapeutics that target MDSCs to enhance antitumor immunity.
Keywords: Cytokines; Immature myeloid cells; Immunotherapy; Inflammation; Macrophages; Myeloid cell cross talk; STAT3; Tumor-induced immune suppression; Tumor-infiltrating lymphocytes; cEBPbeta.
© 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Adeegbe D, Serafini P, Bronte V, Zoso A, Ricordi C, Inverardi L. In vivo induction of myeloid suppressor cells and CD4(+)Foxp3(+) T regulatory cells prolongs skin allograft survival in mice. Cell Transplantation. 2011;20:941–954. - PubMed
-
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. Journal of Immunology. 2001;166:678–689. - PubMed
-
- Annels NE, Shaw VE, Gabitass RF, Billingham L, Corrie P, Eatock M, et al. The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer. Cancer Immunology, Immunotherapy. 2014;63:175–183. - PMC - PubMed
-
- Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

