Acetate Dissimilation and Assimilation in Mycobacterium tuberculosis Depend on Carbon Availability
- PMID: 26216844
- PMCID: PMC4560287
- DOI: 10.1128/JB.00259-15
Acetate Dissimilation and Assimilation in Mycobacterium tuberculosis Depend on Carbon Availability
Abstract
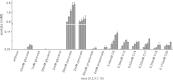
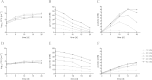
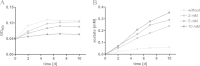
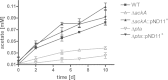
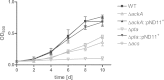
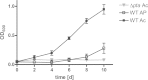
Mycobacterium tuberculosis persists inside granulomas in the human lung. Analysis of the metabolic composition of granulomas from guinea pigs revealed that one of the organic acids accumulating in the course of infection is acetate (B. S. Somashekar, A. G. Amin, C. D. Rithner, J. Troudt, R. Basaraba, A. Izzo, D. C. Crick, and D. Chatterjee, J Proteome Res 10:4186-4195, 2011, doi:http://dx.doi.org/10.1021/pr2003352), which might result either from metabolism of the pathogen or might be provided by the host itself. Our studies characterize a metabolic pathway by which M. tuberculosis generates acetate in the cause of fatty acid catabolism. The acetate formation depends on the enzymatic activities of Pta and AckA. Using actyl coenzyme A (acetyl-CoA) as a substrate, acetyl-phosphate is generated and finally dephosphorylated to acetate, which is secreted into the medium. Knockout mutants lacking either the pta or ackA gene showed significantly reduced acetate production when grown on fatty acids. This effect is even more pronounced when the glyoxylate shunt is blocked, resulting in higher acetate levels released to the medium. The secretion of acetate was followed by an assimilation of the metabolite when other carbon substrates became limiting. Our data indicate that during acetate assimilation, the Pta-AckA pathway acts in concert with another enzymatic reaction, namely, the acetyl-CoA synthetase (Acs) reaction. Thus, acetate metabolism might possess a dual function, mediating an overflow reaction to release excess carbon units and resumption of acetate as a carbon substrate.
Importance: During infection, host-derived lipid components present the major carbon source at the infection site. β-Oxidation of fatty acids results in the formation of acetyl-CoA. In this study, we demonstrate that consumption of fatty acids by Mycobacterium tuberculosis activates an overflow mechanism, causing the pathogen to release excess carbon intermediates as acetate. The Pta-AckA pathway mediating acetate formation proved to be reversible, enabling M. tuberculosis to reutilize the previously secreted acetate as a carbon substrate for metabolism.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. doi:10.1038/31159. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

