Pore dimensions and the role of occupancy in unitary conductance of Shaker K channels
- PMID: 26216859
- PMCID: PMC4516780
- DOI: 10.1085/jgp.201411353
Pore dimensions and the role of occupancy in unitary conductance of Shaker K channels
Abstract
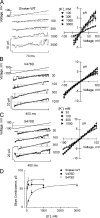
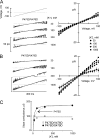
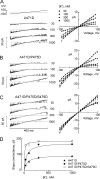
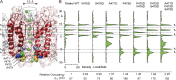
K channels mediate the selective passage of K(+) across the plasma membrane by means of intimate interactions with ions at the pore selectivity filter located near the external face. Despite high conservation of the selectivity filter, the K(+) transport properties of different K channels vary widely, with the unitary conductance spanning a range of over two orders of magnitude. Mutation of Pro475, a residue located at the cytoplasmic entrance of the pore of the small-intermediate conductance K channel Shaker (Pro475Asp (P475D) or Pro475Gln (P475Q)), increases Shaker's reported ∼ 20-pS conductance by approximately six- and approximately threefold, respectively, without any detectable effect on its selectivity. These findings suggest that the structural determinants underlying the diversity of K channel conductance are distinct from the selectivity filter, making P475D and P475Q excellent probes to identify key determinants of the K channel unitary conductance. By measuring diffusion-limited unitary outward currents after unilateral addition of 2 M sucrose to the internal solution to increase its viscosity, we estimated a pore internal radius of capture of ∼ 0.82 Å for all three Shaker variants (wild type, P475D, and P475Q). This estimate is consistent with the internal entrance of the Kv1.2/2.1 structure if the effective radius of hydrated K(+) is set to ∼ 4 Å. Unilateral exposure to sucrose allowed us to estimate the internal and external access resistances together with that of the inner pore. We determined that Shaker resistance resides mainly in the inner cavity, whereas only ∼ 8% resides in the selectivity filter. To reduce the inner resistance, we introduced additional aspartate residues into the internal vestibule to favor ion occupancy. No aspartate addition raised the maximum unitary conductance, measured at saturating [K(+)], beyond that of P475D, suggesting an ∼ 200-pS conductance ceiling for Shaker. This value is approximately one third of the maximum conductance of the large conductance K (BK) channel (the K channel of highest conductance), reducing the energy gap between their K(+) transport rates to ∼ 1 kT. Thus, although Shaker's pore sustains ion translocation as the BK channel's does, higher energetic costs of ion stabilization or higher friction with the ion's rigid hydration cage in its narrower aqueous cavity may entail higher resistance.
© 2015 Díaz-Franulic et al.
Figures


Comment in
-
What keeps Kv channels small? The molecular physiology of modesty.J Gen Physiol. 2015 Aug;146(2):123-7. doi: 10.1085/jgp.201511469. J Gen Physiol. 2015. PMID: 26216857 Free PMC article. No abstract available.
References
-
- Andersen O.S., and Procopio J.. 1980. Ion movement through gramicidin A channels. On the importance of the aqueous diffusion resistance and ion-water interactions. Acta Physiol. Scand. Suppl. 481:27–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

