Sphingomyelinase D inhibits store-operated Ca2+ entry in T lymphocytes by suppressing ORAI current
- PMID: 26216860
- PMCID: PMC4516786
- DOI: 10.1085/jgp.201511359
Sphingomyelinase D inhibits store-operated Ca2+ entry in T lymphocytes by suppressing ORAI current
Abstract
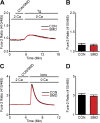
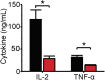
Infections caused by certain bacteria including Mycobacterium tuberculosis and Corynebacterium pseudotuberculosis provoke inflammatory responses characterized by the formation of granulomas with necrotic foci-so-called caseous necrosis. The granulomas of infected animals show prominent infiltration by T lymphocytes, and T cell depletion increases host mortality. Notorious zoonotic C. pseudotuberculosis secretes sphingomyelinase (SMase) D, a phospholipase that cleaves off the choline moiety of sphingomyelin, a phospholipid found primarily in the outer leaflet of host cell plasma membranes. Experimental C. pseudotuberculosis strains that lack SMase D are markedly less infectious and unable to spread in hosts, indicating that this enzyme is a crucial virulence factor for sustaining the caseous lymphadenitis infections caused by this microbe. However, the molecular mechanism by which SMase D helps bacteria evade the host's immune response remains unknown. Here, we find that SMase D inhibits store-operated Ca(2+) entry (SOCE) in human T cells and lowers the production of the SOCE-dependent cytokines interleukin-2, which is critical for T cell growth, proliferation, and differentiation, and tumor necrosis factor α, which is crucial for the formation and maintenance of granulomas in microbial infections. SMase D inhibits SOCE through a previously unknown mechanism, namely, suppression of Orai1 current, rather than through altering gating of voltage-gated K(+) channels. This finding suggests that, whereas certain genetic mutations abolish Orai1 activity causing severe combined immunodeficiency (SCID), bacteria have the ability to suppress Orai1 activity with SMase D to create an acquired, chronic SCID-like condition that allows persistent infection. Thus, in an example of how virulence factors can disrupt key membrane protein function by targeting phospholipids in host cell membranes, our study has uncovered a novel molecular mechanism that bacteria can use to thwart host immunity.
© 2015 Combs and Lu.
Figures




Comment in
-
Sphingomyelin, ORAI1 channels, and cellular Ca2+ signaling.J Gen Physiol. 2015 Sep;146(3):195-200. doi: 10.1085/jgp.201511479. J Gen Physiol. 2015. PMID: 26324674 Free PMC article. No abstract available.
Similar articles
-
Sphingomyelin, ORAI1 channels, and cellular Ca2+ signaling.J Gen Physiol. 2015 Sep;146(3):195-200. doi: 10.1085/jgp.201511479. J Gen Physiol. 2015. PMID: 26324674 Free PMC article. No abstract available.
-
Tuning voltage-gated channel activity and cellular excitability with a sphingomyelinase.J Gen Physiol. 2013 Oct;142(4):367-80. doi: 10.1085/jgp.201310986. Epub 2013 Sep 16. J Gen Physiol. 2013. PMID: 24043861 Free PMC article.
-
TNFR1-induced sphingomyelinase activation modulates TCR signaling by impairing store-operated Ca2+ influx.J Leukoc Biol. 2005 Jul;78(1):266-78. doi: 10.1189/jlb.1003456. Epub 2005 Apr 7. J Leukoc Biol. 2005. PMID: 15817701
-
Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes.Cell Calcium. 2007 Aug;42(2):145-56. doi: 10.1016/j.ceca.2007.03.007. Epub 2007 Jun 18. Cell Calcium. 2007. PMID: 17572487 Review.
-
Alternative forms of the store-operated calcium entry mediators, STIM1 and Orai1.Curr Top Membr. 2013;71:109-23. doi: 10.1016/B978-0-12-407870-3.00005-6. Curr Top Membr. 2013. PMID: 23890113 Review.
Cited by
-
The Role of Lipids in CRAC Channel Function.Biomolecules. 2022 Feb 23;12(3):352. doi: 10.3390/biom12030352. Biomolecules. 2022. PMID: 35327543 Free PMC article. Review.
-
Insight of Genus Corynebacterium: Ascertaining the Role of Pathogenic and Non-pathogenic Species.Front Microbiol. 2017 Oct 12;8:1937. doi: 10.3389/fmicb.2017.01937. eCollection 2017. Front Microbiol. 2017. PMID: 29075239 Free PMC article. Review.
-
Bacterial Sphingomyelinases and Phospholipases as Virulence Factors.Microbiol Mol Biol Rev. 2016 Jun 15;80(3):597-628. doi: 10.1128/MMBR.00082-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27307578 Free PMC article. Review.
-
Sphingomyelin, ORAI1 channels, and cellular Ca2+ signaling.J Gen Physiol. 2015 Sep;146(3):195-200. doi: 10.1085/jgp.201511479. J Gen Physiol. 2015. PMID: 26324674 Free PMC article. No abstract available.
-
Regulation of Membrane Calcium Transport Proteins by the Surrounding Lipid Environment.Biomolecules. 2019 Sep 20;9(10):513. doi: 10.3390/biom9100513. Biomolecules. 2019. PMID: 31547139 Free PMC article. Review.
References
-
- Breittmayer J.P., Bernard A., and Aussel C.. 1994. Regulation by sphingomyelinase and sphingosine of Ca2+ signals elicited by CD3 monoclonal antibody, thapsigargin, or ionomycin in the Jurkat T cell line. J. Biol. Chem. 269:5054–5058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

