Endocytosis-dependent coordination of multiple actin regulators is required for wound healing
- PMID: 26216900
- PMCID: PMC4523608
- DOI: 10.1083/jcb.201411037
Endocytosis-dependent coordination of multiple actin regulators is required for wound healing
Erratum in
-
Endocytosis-dependent coordination of multiple actin regulators is required for wound healing.J Cell Biol. 2015 Aug 17;210(4):677-9. doi: 10.1083/jcb.20141103707282015c. Epub 2015 Aug 10. J Cell Biol. 2015. PMID: 26261184 Free PMC article. No abstract available.
Abstract
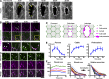
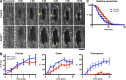
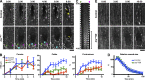
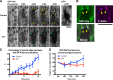
The ability to heal wounds efficiently is essential for life. After wounding of an epithelium, the cells bordering the wound form dynamic actin protrusions and/or a contractile actomyosin cable, and these actin structures drive wound closure. Despite their importance in wound healing, the molecular mechanisms that regulate the assembly of these actin structures at wound edges are not well understood. In this paper, using Drosophila melanogaster embryos, we demonstrate that Diaphanous, SCAR, and WASp play distinct but overlapping roles in regulating actin assembly during wound healing. Moreover, we show that endocytosis is essential for wound edge actin assembly and wound closure. We identify adherens junctions (AJs) as a key target of endocytosis during wound healing and propose that endocytic remodeling of AJs is required to form "signaling centers" along the wound edge that control actin assembly. We conclude that coordination of actin assembly, AJ remodeling, and membrane traffic is required for the construction of a motile leading edge during wound healing.
© 2015 Matsubayashi et al.
Figures




Similar articles
-
Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis.Curr Biol. 2008 Nov 11;18(21):1639-48. doi: 10.1016/j.cub.2008.09.063. Epub 2008 Oct 30. Curr Biol. 2008. PMID: 18976911
-
Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability.Curr Biol. 2008 Nov 11;18(21):1631-8. doi: 10.1016/j.cub.2008.09.029. Epub 2008 Oct 30. Curr Biol. 2008. PMID: 18976918
-
Polarized E-cadherin endocytosis directs actomyosin remodeling during embryonic wound repair.J Cell Biol. 2015 Aug 31;210(5):801-16. doi: 10.1083/jcb.201501076. Epub 2015 Aug 24. J Cell Biol. 2015. PMID: 26304727 Free PMC article.
-
Tension (re)builds: Biophysical mechanisms of embryonic wound repair.Mech Dev. 2017 Apr;144(Pt A):43-52. doi: 10.1016/j.mod.2016.11.004. Epub 2016 Dec 15. Mech Dev. 2017. PMID: 27989746 Review.
-
Ever-expanding network of dynamin-interacting proteins.Mol Neurobiol. 2006 Oct;34(2):129-36. doi: 10.1385/MN:34:2:129. Mol Neurobiol. 2006. PMID: 17220534 Review.
Cited by
-
Actin polymerization state regulates osteogenic differentiation in human adipose-derived stem cells.Cell Mol Biol Lett. 2021 Apr 15;26(1):15. doi: 10.1186/s11658-021-00259-8. Cell Mol Biol Lett. 2021. PMID: 33858321 Free PMC article.
-
Wound Repair of the Cell Membrane: Lessons from Dictyostelium Cells.Cells. 2024 Feb 14;13(4):341. doi: 10.3390/cells13040341. Cells. 2024. PMID: 38391954 Free PMC article. Review.
-
The actin polymerization factor Diaphanous and the actin severing protein Flightless I collaborate to regulate sarcomere size.Dev Biol. 2021 Jan 1;469:12-25. doi: 10.1016/j.ydbio.2020.09.014. Epub 2020 Sep 25. Dev Biol. 2021. PMID: 32980309 Free PMC article.
-
Crumbs is an essential regulator of cytoskeletal dynamics and cell-cell adhesion during dorsal closure in Drosophila.Elife. 2015 Nov 6;4:e07398. doi: 10.7554/eLife.07398. Elife. 2015. PMID: 26544546 Free PMC article.
-
Polarity during tissue repair, a multiscale problem.Curr Opin Cell Biol. 2020 Feb;62:31-36. doi: 10.1016/j.ceb.2019.07.015. Epub 2019 Sep 9. Curr Opin Cell Biol. 2020. PMID: 31514044 Free PMC article. Review.
References
-
- Afshar K., Stuart B., and Wasserman S.A.. 2000. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 127:1887–1897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

