Rational design of antibodies targeting specific epitopes within intrinsically disordered proteins
- PMID: 26216991
- PMCID: PMC4538631
- DOI: 10.1073/pnas.1422401112
Rational design of antibodies targeting specific epitopes within intrinsically disordered proteins
Abstract
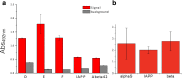
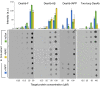
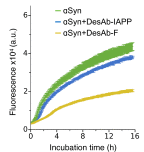
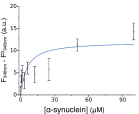
Antibodies are powerful tools in life sciences research, as well as in diagnostic and therapeutic applications, because of their ability to bind given molecules with high affinity and specificity. Using current methods, however, it is laborious and sometimes difficult to generate antibodies to target specific epitopes within a protein, in particular if these epitopes are not effective antigens. Here we present a method to rationally design antibodies to enable them to bind virtually any chosen disordered epitope in a protein. The procedure consists in the sequence-based design of one or more complementary peptides targeting a selected disordered epitope and the subsequent grafting of such peptides on an antibody scaffold. We illustrate the method by designing six single-domain antibodies to bind different epitopes within three disease-related intrinsically disordered proteins and peptides (α-synuclein, Aβ42, and IAPP). Our results show that all these designed antibodies bind their targets with good affinity and specificity. As an example of an application, we show that one of these antibodies inhibits the aggregation of α-synuclein at substoichiometric concentrations and that binding occurs at the selected epitope. Taken together, these results indicate that the design strategy that we propose makes it possible to obtain antibodies targeting given epitopes in disordered proteins or protein regions.
Keywords: complementary peptides; protein aggregation; protein design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







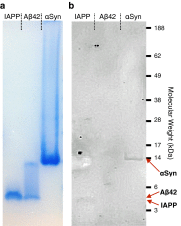
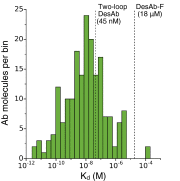
References
-
- Carter PJ. Introduction to current and future protein therapeutics: A protein engineering perspective. Exp Cell Res. 2011;317(9):1261–1269. - PubMed
-
- Elvin JG, Couston RG, van der Walle CF. Therapeutic antibodies: Market considerations, disease targets and bioprocessing. Int J Pharm. 2013;440(1):83–98. - PubMed
-
- Goodman M. Market watch: Sales of biologics to show robust growth through to 2013. Nat Rev Drug Discov. 2009;8(11):837–837. - PubMed
-
- Leader B, Baca QJ, Golan DE. Protein therapeutics: A summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1):21–39. - PubMed
-
- Pavlou AK, Reichert JM. Recombinant protein therapeutics: Success rates, market trends and values to 2010. Nat Biotechnol. 2004;22(12):1513–1519. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

