Potential protective effects of Clostridium butyricum on experimental gastric ulcers in mice
- PMID: 26217085
- PMCID: PMC4507103
- DOI: 10.3748/wjg.v21.i27.8340
Potential protective effects of Clostridium butyricum on experimental gastric ulcers in mice
Abstract
Aim: To investigate the effects of Clostridium butyricum (C. butyricum) on experimental gastric ulcers (GUs) induced by alcohol, restraint cold stress, or pyloric ligation in mice, respectively.
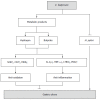
Methods: One hundred and twenty mice were randomly allocated into three types of gastric ulcer models (n = 40 each), induced by alcohol, restraint cold stress, or pyloric ligation. In each GU model, 40 mice were allocated into four groups (n = 10 each): the sham control group; model group (GU induction without pretreatment); C. butyricum group (GU induction with C. butyricum pretreatment); and Omeprazole group (GU induction with Omeprazole pretreatment). The effects of C. butyricum were evaluated by examining the histological changes in the gastric mucosal erosion area, the activities of superoxide dismutase (SOD) and catalase (CAT), the level of malondialdehyde (MDA), and the contents of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, leukotriene B4 (LTB4) and 6-keto-PGF-1α (degradation product of PGI2) in the gastric tissue.
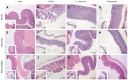
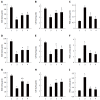
Results: Our data showed that C. butyricum significantly reduced the gastric mucosal injury area and ameliorated the pathological conditions of the gastric mucosa. C. butyricum not only minimized the decreases in activity of SOD and CAT, but also reduced the level of MDA in all three GU models used in this study. The accumulation of IL1-β, TNF-α and LBT4 decreased, while 6-keto-PGF-1α increased with pretreatment by C. butyricum in all three GU models.
Conclusion: Our data demonstrated the protective effects of pretreatment with C. butyricum on anti-oxidation and anti-inflammation in different types of GU models in mice. Further studies are needed to explore its potential clinical benefits.
Keywords: Clostridium butyricum; Gastric ulcer; Inflammation; Oxidative stress; Probiotics.
Figures




Similar articles
-
Pomegranate as a natural remedy for gastric ulcers prevention: a review of its gastroprotective mechanisms and pharmacological benefits.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):6675-6690. doi: 10.1007/s00210-025-03822-8. Epub 2025 Jan 31. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39888366 Review.
-
Effect of methanolic extract of Pongamia pinnata Linn seed on gastro-duodenal ulceration and mucosal offensive and defensive factors in rats.Indian J Exp Biol. 2009 Aug;47(8):649-59. Indian J Exp Biol. 2009. PMID: 19775071
-
Protective effects of alginate-chitosan microspheres loaded with alkaloids from Coptis chinensis Franch. and Evodia rutaecarpa (Juss.) Benth. (Zuojin Pill) against ethanol-induced acute gastric mucosal injury in rats.Drug Des Devel Ther. 2015 Nov 19;9:6151-65. doi: 10.2147/DDDT.S96056. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26640368 Free PMC article.
-
The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice.Food Funct. 2017 Nov 15;8(11):4042-4052. doi: 10.1039/c7fo00355b. Food Funct. 2017. PMID: 28933492
-
The Role of Triterpenoids in Gastric Ulcer: Mechanisms and Therapeutic Potentials.Int J Mol Sci. 2025 Mar 31;26(7):3237. doi: 10.3390/ijms26073237. Int J Mol Sci. 2025. PMID: 40244034 Free PMC article. Review.
Cited by
-
Prevotella histicola suppresses ferroptosis to mitigate ethanol-induced gastric mucosal lesions in mice.BMC Complement Med Ther. 2023 Apr 14;23(1):118. doi: 10.1186/s12906-023-03946-5. BMC Complement Med Ther. 2023. PMID: 37060026 Free PMC article.
-
Pomegranate as a natural remedy for gastric ulcers prevention: a review of its gastroprotective mechanisms and pharmacological benefits.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):6675-6690. doi: 10.1007/s00210-025-03822-8. Epub 2025 Jan 31. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39888366 Review.
-
The effect of dietary supplementation with Clostridium butyricum on the growth performance, immunity, intestinal microbiota and disease resistance of tilapia (Oreochromis niloticus).PLoS One. 2019 Dec 9;14(12):e0223428. doi: 10.1371/journal.pone.0223428. eCollection 2019. PLoS One. 2019. PMID: 31815958 Free PMC article.
-
Effects of dietary Clostridium butyricum addition to sows in late gestation and lactation on reproductive performance and intestinal microbiota1.J Anim Sci. 2019 Jul 30;97(8):3426-3439. doi: 10.1093/jas/skz186. J Anim Sci. 2019. PMID: 31233597 Free PMC article.
-
The Use of Pistacia Lentiscus Chia Resin Versus Omeprazole in Protecting Male Rats Peptic Mucosa Against Cold Restraint Stress.J Crit Care Med (Targu Mures). 2020 May 6;6(2):100-110. doi: 10.2478/jccm-2020-0018. eCollection 2020 Apr. J Crit Care Med (Targu Mures). 2020. PMID: 32426516 Free PMC article.
References
-
- Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88:1547–1565. - PubMed
-
- den Hollander WJ, Kuipers EJ. Current pharmacotherapy options for gastritis. Expert Opin Pharmacother. 2012;13:2625–2636. - PubMed
-
- Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163–169. - PubMed
-
- Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744–2749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

