Active mechanistic target of rapamycin plays an ancillary rather than essential role in zebrafish CNS axon regeneration
- PMID: 26217179
- PMCID: PMC4493654
- DOI: 10.3389/fncel.2015.00251
Active mechanistic target of rapamycin plays an ancillary rather than essential role in zebrafish CNS axon regeneration
Abstract
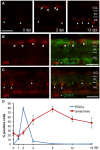
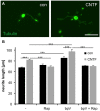
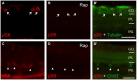
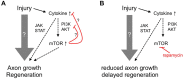
The developmental decrease of the intrinsic regenerative ability of the mammalian central nervous system (CNS) is associated with reduced activity of mechanistic target of rapamycin (mTOR) in mature neurons such as retinal ganglion cells (RGCs). While mTOR activity is further decreased upon axonal injury, maintenance of its pre-injury level, for instance by genetic deletion of the phosphatase and tensin homolog (PTEN), markedly promotes axon regeneration in mammals. The current study now addressed the question whether active mTOR might generally play a central role in axon regeneration by analyzing its requirement in regeneration-competent zebrafish. Remarkably, regulation of mTOR activity after optic nerve injury in zebrafish is fundamentally different compared to mammals. Hardly any activity was detected in naïve RGCs, whereas it was markedly increased upon axotomy in vivo as well as in dissociated cell cultures. After a short burst, mTOR activity was quickly attenuated, which is contrary to the requirements for axon regeneration in mammals. Surprisingly, mTOR activity was not essential for axonal growth per se, but correlated with cytokine- and PTEN inhibitor-induced neurite extension in vitro. Moreover, inhibition of mTOR using rapamycin significantly reduced axon regeneration in vivo and compromised functional recovery after optic nerve injury. Therefore, axotomy-induced mTOR activity is involved in CNS axon regeneration in zebrafish similar to mammals, although it plays an ancillary rather than essential role in this regeneration-competent species.
Keywords: mTOR; optic nerve regeneration; pS6; rapamycin; zebrafish.
Figures


References
-
- Bernhardt R. R., Tongiorgi E., Anzini P., Schachner M. (1996). Increased expression of specific recognition molecules by retinal ganglion cells and by optic pathway glia accompanies the successful regeneration of retinal axons in adult zebrafish. J. Comp. Neurol. 376, 253–264. 10.1002/(sici)1096-9861(19961209)376:2<253::aid-cne7>3.0.co;2-2 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

