Regulated phosphorylation of the K-Cl cotransporter KCC3 is a molecular switch of intracellular potassium content and cell volume homeostasis
- PMID: 26217182
- PMCID: PMC4496573
- DOI: 10.3389/fncel.2015.00255
Regulated phosphorylation of the K-Cl cotransporter KCC3 is a molecular switch of intracellular potassium content and cell volume homeostasis
Abstract
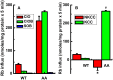
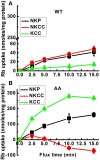
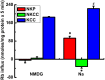
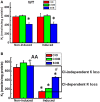
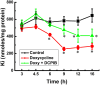
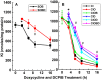
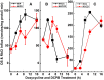
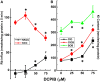
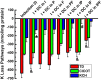
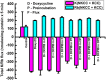
The defense of cell volume against excessive shrinkage or swelling is a requirement for cell function and organismal survival. Cell swelling triggers a coordinated homeostatic response termed regulatory volume decrease (RVD), resulting in K(+) and Cl(-) efflux via activation of K(+) channels, volume-regulated anion channels (VRACs), and the K(+)-Cl(-) cotransporters, including KCC3. Here, we show genetic alanine (Ala) substitution at threonines (Thr) 991 and 1048 in the KCC3a isoform carboxyl-terminus, preventing inhibitory phosphorylation at these sites, not only significantly up-regulates KCC3a activity up to 25-fold in normally inhibitory isotonic conditions, but is also accompanied by reversal of activity of the related bumetanide-sensitive Na(+)-K(+)-2Cl(-) cotransporter isoform 1 (NKCC1). This results in a rapid (<10 min) and significant (>90%) reduction in intracellular K(+) content (Ki) via both Cl-dependent (KCC3a + NKCC1) and Cl-independent [DCPIB (VRAC inhibitor)-sensitive] pathways, which collectively renders cells less prone to acute swelling in hypotonic osmotic stress. Together, these data demonstrate the phosphorylation state of Thr991/Thr1048 in KCC3a encodes a potent switch of transporter activity, Ki homeostasis, and cell volume regulation, and reveal novel observations into the functional interaction among ion transport molecules involved in RVD.
Keywords: K-Cl cotransporters; KCC3; NKCC1; SPAK; cell volume homeostasis; cerebral edema; neurodegeneration; regulatory volume decrease.
Figures

References
-
- Adragna N. C., Fonseca P., Lauf P. K. (1994). Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood 83, 553–560. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

