Muscle wasting in myotonic dystrophies: a model of premature aging
- PMID: 26217220
- PMCID: PMC4496580
- DOI: 10.3389/fnagi.2015.00125
Muscle wasting in myotonic dystrophies: a model of premature aging
Abstract
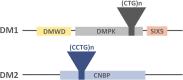
Myotonic dystrophy type 1 (DM1 or Steinert's disease) and type 2 (DM2) are multisystem disorders of genetic origin. Progressive muscular weakness, atrophy and myotonia are the most prominent neuromuscular features of these diseases, while other clinical manifestations such as cardiomyopathy, insulin resistance and cataracts are also common. From a clinical perspective, most DM symptoms are interpreted as a result of an accelerated aging (cataracts, muscular weakness and atrophy, cognitive decline, metabolic dysfunction, etc.), including an increased risk of developing tumors. From this point of view, DM1 could be described as a progeroid syndrome since a notable age-dependent dysfunction of all systems occurs. The underlying molecular disorder in DM1 consists of the existence of a pathological (CTG) triplet expansion in the 3' untranslated region (UTR) of the Dystrophia Myotonica Protein Kinase (DMPK) gene, whereas (CCTG)n repeats in the first intron of the Cellular Nucleic acid Binding Protein/Zinc Finger Protein 9 (CNBP/ZNF9) gene cause DM2. The expansions are transcribed into (CUG)n and (CCUG)n-containing RNA, respectively, which form secondary structures and sequester RNA-binding proteins, such as the splicing factor muscleblind-like protein (MBNL), forming nuclear aggregates known as foci. Other splicing factors, such as CUGBP, are also disrupted, leading to a spliceopathy of a large number of downstream genes linked to the clinical features of these diseases. Skeletal muscle regeneration relies on muscle progenitor cells, known as satellite cells, which are activated after muscle damage, and which proliferate and differentiate to muscle cells, thus regenerating the damaged tissue. Satellite cell dysfunction seems to be a common feature of both age-dependent muscle degeneration (sarcopenia) and muscle wasting in DM and other muscle degenerative diseases. This review aims to describe the cellular, molecular and macrostructural processes involved in the muscular degeneration seen in DM patients, highlighting the similarities found with muscle aging.
Keywords: aging; muscle wasting; myotonic dystrophy; sarcopenia; satellite cells.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

