Altered NK cell function in obese healthy humans
- PMID: 26217516
- PMCID: PMC4511543
- DOI: 10.1186/s40608-014-0033-1
Altered NK cell function in obese healthy humans
Abstract
Background: Obesity is associated with an elevated risk for several types of cancer and thus a major health hazard. However, the mechanism between overweight and cancer susceptibility is still elusive. Leptin, mainly produced by adipocytes links food intake and energy expenditure. In addition, recent studies have shown an immunomodulatory impact of leptin on NK cells. The purpose of the present study was to investigate whether leptin stimulation of NK cells from obese humans leads to altered functions as compared to NK cells from lean subjects. On the basis of body mass index 20 healthy individuals were classified in two groups: normal weight (<25 kg/m(2)) and obese (>30 kg/m(2)). Peripheral blood mononuclear cells (PBMC) were isolated from blood samples. We used flow cytometry to assess differences in phenotype and activity markers (CD107a, CD178 and TRAIL) of PBMCs between both groups. Furthermore, we determined after short-term in vitro leptin stimulation the phosphorylation of JAK2, downstream target of the intracellular signaling cascade of the leptin receptor, by Western Blotting and numbers of NK-cell-tumor-cell-conjugates as well as Granzyme(+) and IFN-γ(+) NK cells by flow cytometry. Finally, the proliferative capacity of control and long-term (7 days) leptin-stimulated NK cells was examined.
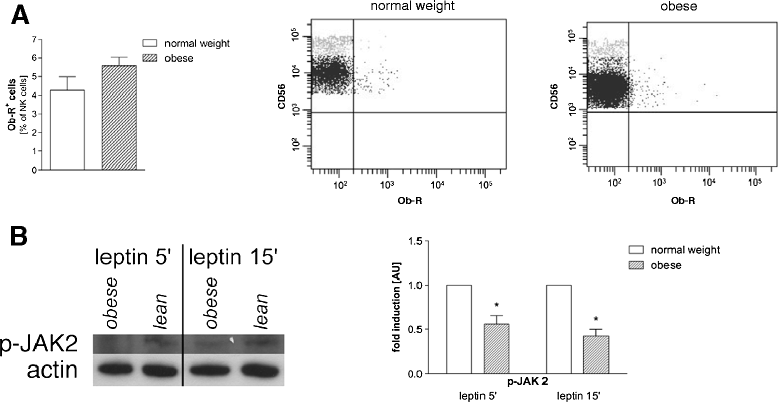
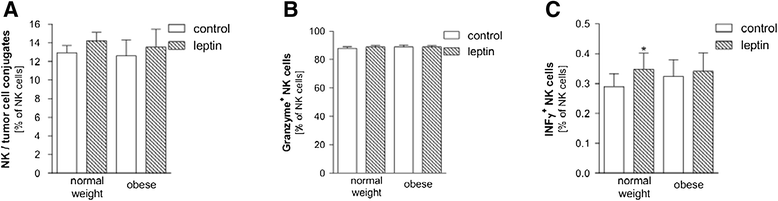
Results: As opposed to similar NK cell counts, the number of CD3(+)CD56(+) cells was significantly lower in obese compared to lean subjects. Human NK cells express the leptin receptor (Ob-R). For further determination of Ob-R, intracellular target proteins of PBMCs were investigated by Western Blotting. Phosphorylation of JAK2 was lower in obese as compared to normal weight subjects. Furthermore, significantly lower levels of TNF-related apoptosis-inducing ligand (TRAIL) as an NK cell functional marker in obese subjects were found. In vitro leptin stimulation resulted in a higher production of interferon-γ in NK cells of normal weight subjects. Interestingly, long-term leptin stimulation had no significant influence on numbers of proliferating NK cells.
Conclusions: NK cells from obese healthy humans show functional deficits and altered responses after in vitro leptin challenge.
Keywords: CD107a; Immunity; Leptin; Natural killer (NK) cells; Ob-R; Obesity; TRAIL.
Figures



References
-
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68(4):437–46. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

