Time-resolved infrared spectroscopic techniques as applied to channelrhodopsin
- PMID: 26217670
- PMCID: PMC4493399
- DOI: 10.3389/fmolb.2015.00038
Time-resolved infrared spectroscopic techniques as applied to channelrhodopsin
Abstract
Among optogenetic tools, channelrhodopsins, the light gated ion channels of the plasma membrane from green algae, play the most important role. Properties like channel selectivity, timing parameters or color can be influenced by the exchange of selected amino acids. Although widely used, in the field of neurosciences for example, there is still little known about their photocycles and the mechanism of ion channel gating and conductance. One of the preferred methods for these studies is infrared spectroscopy since it allows observation of proteins and their function at a molecular level and in near-native environment. The absorption of a photon in channelrhodopsin leads to retinal isomerization within femtoseconds, the conductive states are reached in the microsecond time scale and the return into the fully dark-adapted state may take more than minutes. To be able to cover all these time regimes, a range of different spectroscopical approaches are necessary. This mini-review focuses on time-resolved applications of the infrared technique to study channelrhodopsins and other light triggered proteins. We will discuss the approaches with respect to their suitability to the investigation of channelrhodopsin and related proteins.
Keywords: FTIR; IR-spectrometer; channelrhodopsin; infrared spectroscopy; retinal proteins; time-resolved spectroscopy.
Figures
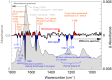
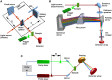
References
-
- Alexandre M. T., Domratcheva T., Bonetti C., Van Wilderen L. J., Van Grondelle R., Groot M. L., et al. (2009). Primary reactions of the LOV2 domain of phototropin studied with ultrafast mid-infrared spectroscopy and quantum chemistry. Biophys. J. 97, 227–237. 10.1016/j.bpj.2009.01.066 - DOI - PMC - PubMed
-
- Andrews S. S., Boxer S. G. (2001). Analysis of noise for rapid-scan and step-scan methods of FT-IR difference spectroscopy. Appl. Spectrosc. 55, 1161–1165. 10.1366/0003702011953414 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

