Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors
- PMID: 26217876
- PMCID: PMC4519988
- DOI: 10.1002/cncr.29587
Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors
Abstract
Background: Chronic use of tyrosine kinase inhibitors (TKIs) may lead to previously unrecognized adverse events. This study evaluated the incidence of acute kidney injury (AKI) and chronic kidney disease (CKD) in chronic-phase (CP) chronic myeloid leukemia (CML) patients treated with imatinib, dasatinib, and nilotinib.
Methods: Four hundred sixty-eight newly diagnosed CP CML patients treated with TKIs were analyzed. The molecular and cytogenetic response data, creatinine, and glomerular filtration rate (GFR) were followed from the start of therapy to the last follow-up (median, 52 months). GFR was estimated with the Modification of Diet in Renal Disease equation.
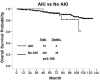
Results: Nineteen patients (4%) had TKI-associated AKI. Imatinib was associated with a higher incidence of AKI in comparison with dasatinib and nilotinib (P = .014). Fifty-eight patients (14%) developed CKD while they were receiving a TKI; 49 of these patients (84%) did so while they were being treated with imatinib (P < .001). Besides imatinib, age, a history of hypertension, and diabetes mellitus were also associated with the development of CKD. In patients with no CKD at the baseline, imatinib was shown to reduce GFR over time. Interestingly, imatinib did not cause a significant decline in the GFRs of patients with a history of CKD. Imatinib, dasatinib, and nilotinib increased the mean GFR after 3 months of treatment, and nilotinib led with the most significant increase (P < .001). AKI or CKD had no significant impact on overall cytogenetic and molecular response rates or survival.
Conclusions: The administration of TKIs may be safe in the setting of CKD in CP CML patients, but close monitoring is still warranted.
Keywords: chronic myeloid leukemia (CML); dasatinib; glomerular filtration rate changes; imatinib; kidney injury; nilotinib; outcome; tyrosine kinase inhibitor.
© 2015 American Cancer Society.
Figures










References
-
- O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. - PubMed
-
- Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. - PubMed
-
- Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

